|
Predictive Probability Of Success
Predictive probability of success (PPOS) is a statistics concept commonly used in the pharmaceutical industry including by health authorities to support decision making. In clinical trials, PPOS is the probability of observing a success in the future based on existing data. It is one type of probability of success. A Bayesian means by which the PPOS can be determined is through integrating the data's likelihood over possible future responses (posterior distribution). Types of PPOS * Classification based on type of end point: Normal, binary, time to event. * Classification based on the relationship between the trial providing data and the trial to be predicted # Cross trial PPOS: using data from one trial to predict the other trial # Within trial PPOS: using data at interim analysis to predict the same trial at final analysis * Classification based on the relationship between the end point(s) with data and the end point to be predicted # 1 to 1 PPOS: using one end point to predict ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Industry
The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered to patients (or self-administered), with the aim to cure them, vaccinate them, or alleviate symptoms. Pharmaceutical companies may deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations that govern the patenting, testing, safety, efficacy using drug testing and marketing of drugs. The global pharmaceuticals market produced treatments worth $1,228.45 billion in 2020 and showed a compound annual growth rate (CAGR) of 1.8%. History Mid-1800s – 1945: From botanicals to the first synthetic drugs The modern era of pharmaceutical industry began with local apothecaries that expanded from their traditional role of distributing botanical drugs such as morphine and quinine to wholesale manufacture in the mid-1800s, and from discoveries resulting from applied research. Intentional drug ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bayesian Probability
Bayesian probability is an Probability interpretations, interpretation of the concept of probability, in which, instead of frequentist probability, frequency or propensity probability, propensity of some phenomenon, probability is interpreted as reasonable expectation representing a state of knowledge or as quantification of a personal belief. The Bayesian interpretation of probability can be seen as an extension of propositional logic that enables reasoning with Hypothesis, hypotheses; that is, with propositions whose truth value, truth or falsity is unknown. In the Bayesian view, a probability is assigned to a hypothesis, whereas under frequentist inference, a hypothesis is typically tested without being assigned a probability. Bayesian probability belongs to the category of evidential probabilities; to evaluate the probability of a hypothesis, the Bayesian probabilist specifies a prior probability. This, in turn, is then updated to a posterior probability in the light of new, re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
False Positive
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test result incorrectly indicates the absence of a condition when it is actually present. These are the two kinds of errors in a binary test, in contrast to the two kinds of correct result (a and a ). They are also known in medicine as a false positive (or false negative) diagnosis, and in statistical classification as a false positive (or false negative) error. In statistical hypothesis testing the analogous concepts are known as type I and type II errors, where a positive result corresponds to rejecting the null hypothesis, and a negative result corresponds to not rejecting the null hypothesis. The terms are often used interchangeably, but there are differences in detail and interpretation due to the differences between medical testing and statist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
False Negative
A false positive is an error in binary classification in which a test result incorrectly indicates the presence of a condition (such as a disease when the disease is not present), while a false negative is the opposite error, where the test result incorrectly indicates the absence of a condition when it is actually present. These are the two kinds of errors in a binary test, in contrast to the two kinds of correct result (a and a ). They are also known in medicine as a false positive (or false negative) diagnosis, and in statistical classification as a false positive (or false negative) error. In statistical hypothesis testing the analogous concepts are known as type I and type II errors, where a positive result corresponds to rejecting the null hypothesis, and a negative result corresponds to not rejecting the null hypothesis. The terms are often used interchangeably, but there are differences in detail and interpretation due to the differences between medical testing and statist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type I Error
In statistical hypothesis testing, a type I error is the mistaken rejection of an actually true null hypothesis (also known as a "false positive" finding or conclusion; example: "an innocent person is convicted"), while a type II error is the failure to reject a null hypothesis that is actually false (also known as a "false negative" finding or conclusion; example: "a guilty person is not convicted"). Much of statistical theory revolves around the minimization of one or both of these errors, though the complete elimination of either is a statistical impossibility if the outcome is not determined by a known, observable causal process. By selecting a low threshold (cut-off) value and modifying the alpha (α) level, the quality of the hypothesis test can be increased. The knowledge of type I errors and type II errors is widely used in medical science, biometrics and computer science. Intuitively, type I errors can be thought of as errors of ''commission'', i.e. the researcher unluck ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apples And Oranges
A comparison of apples and oranges occurs when two items or groups of items are compared that cannot be practically compared, typically because of inherent, fundamental and/or qualitative differences between the items. The idiom, ''comparing apples and oranges'', refers to the apparent differences between items which are popularly thought to be incomparable or incommensurable, such as apples and oranges. The idiom may also be used to indicate that a false analogy has been made between two items, such as where an ''apple'' is faulted for not being a good ''orange''. Variants The idiom is not unique to English. In Quebec French, it may take the form (to compare apples with oranges), while in European French the idiom says (to compare apples and pears) or (to compare cabbages and carrots). In Latin American Spanish, it is usually (comparing potatoes and sweet potatoes) or commonly for all varieties of Spanish (comparing pears with apples). In some other languages the ter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can vary i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Credible Interval
In Bayesian statistics, a credible interval is an interval within which an unobserved parameter value falls with a particular probability. It is an interval in the domain of a posterior probability distribution or a predictive distribution. The generalisation to multivariate problems is the credible region. Credible intervals are analogous to confidence intervals and confidence regions in frequentist statistics, although they differ on a philosophical basis: Bayesian intervals treat their bounds as fixed and the estimated parameter as a random variable, whereas frequentist confidence intervals treat their bounds as random variables and the parameter as a fixed value. Also, Bayesian credible intervals use (and indeed, require) knowledge of the situation-specific prior distribution, while the frequentist confidence intervals do not. For example, in an experiment that determines the distribution of possible values of the parameter \mu, if the subjective probability that \mu lie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
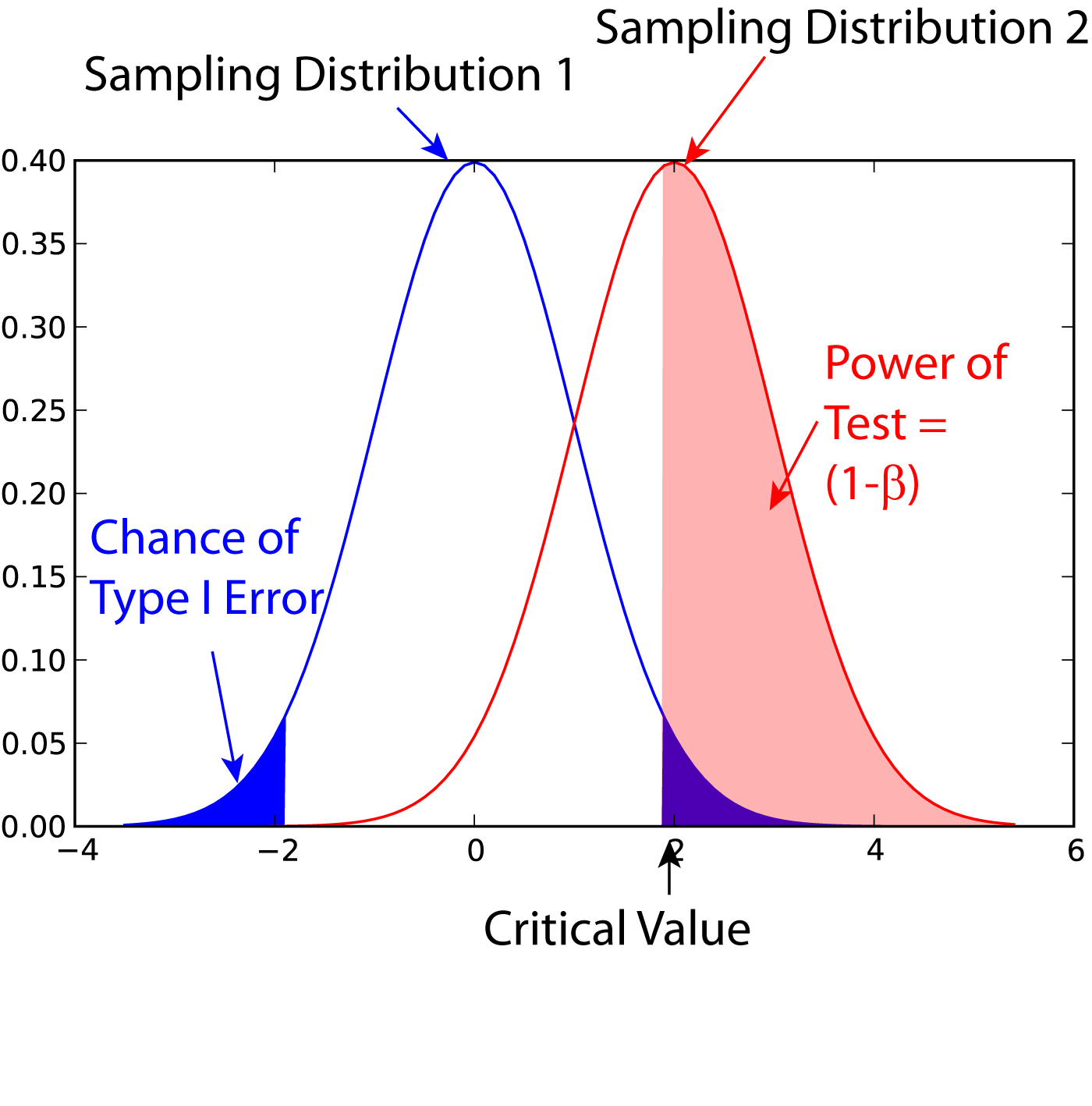
Statistical Power
In statistics, the power of a binary hypothesis test is the probability that the test correctly rejects the null hypothesis (H_0) when a specific alternative hypothesis (H_1) is true. It is commonly denoted by 1-\beta, and represents the chances of a true positive detection conditional on the actual existence of an effect to detect. Statistical power ranges from 0 to 1, and as the power of a test increases, the probability \beta of making a type II error by wrongly failing to reject the null hypothesis decreases. Notation This article uses the following notation: * ''β'' = probability of a Type II error, known as a "false negative" * 1 − ''β'' = probability of a "true positive", i.e., correctly rejecting the null hypothesis. "1 − ''β''" is also known as the power of the test. * ''α'' = probability of a Type I error, known as a "false positive" * 1 − ''α'' = probability of a "true negative", i.e., correctly not rejecting the null hypothesis Description For a ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Authority
Between 1996 and 2002 the English National Health Service was organised under the following health authorities. In 2002 the health authorities were reorganised and their boundaries changed to constitute 28 strategic health authorities, which were reduced in number to 10 in 2006. Prior to 1996 the service was organised according to regional health authorities. ‡ also included part of High Peak in Derbyshire (East Midlands) These Health Authorities were established in 1996. There were a few changes between then and the final form shown above. There were originally separate authorities for Barnet and Enfield & Haringey, for Bexley & Greenwich and Bromley, for East & North Hertfordshire and South Hertfordshire, and for the Isle of Wight & Portsmouth and South-East Hampshire. Also, the area of Norfolk and Cambridgeshire was partitioned between three authorities : Cambridge & Huntingdon, East Norfolk, and North West Anglia. North West Anglia included from Cambridgeshire: Pete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frequentist
Frequentist inference is a type of statistical inference based in frequentist probability, which treats “probability” in equivalent terms to “frequency” and draws conclusions from sample-data by means of emphasizing the frequency or proportion of findings in the data. Frequentist-inference underlies frequentist statistics, in which the well-established methodologies of statistical hypothesis testing and confidence intervals are founded. History of frequentist statistics The history of frequentist statistics is more recent than its prevailing philosophical rival, Bayesian statistics. Frequentist statistics were largely developed in the early 20th century and have recently developed to become the dominant paradigm in inferential statistics, while Bayesian statistics were invented in the 19th century. Despite this dominance, there is no agreement as to whether frequentism is better than Bayesian statistics, with a vocal minority of professionals studying statistical in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Endpoint
Clinical endpoints or clinical outcomes are Outcome measure, outcome measures referring to occurrence of disease, symptom, Medical sign, sign or laboratory abnormality constituting a target outcome in clinical trial, clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a ''humane (clinical) endpoint''. The primary endpoint of a clinical trial is the endpoint for which the trial is statistical power, powered. Secondary endpoints are additional endpoints, preferably also pre-specified, for which the trial may not be powered. Surrogate endpoint, Surrogate endpoints are trial endpoints that have outcomes that substitute for a clinical endpoint, often because studying the clinical endpoint is difficult, for example using an increase in blood pressure as a surrogate for death by cardiovascular disease, where strong evidence of a causal link exists. Scope In a general sens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_(6982162417).jpg)

.jpg)