|
Polycrystal
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel longulites. Structure The orientation of crystallites can be random with no preferred direction, called random texture, or directed, possibly due to growth and processing conditions. While the structure of a (single) crystal is highly ordered and its lattice is continuous and unbroken, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in-between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are polycrystalline, made of a large number crystallites held together by thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Texture (chemistry)
In physical chemistry and materials science, texture is the distribution of crystallographic orientations of a polycrystalline sample (it is also part of the geological fabric (geology), fabric). A sample in which these orientations are fully random is said to have no distinct texture. If the crystallographic orientations are not random, but have some preferred orientation, then the sample has a weak, moderate or strong texture. The degree is dependent on the percentage of crystals having the preferred orientation. Texture is seen in almost all engineered materials, and can have a great influence on materials properties. The texture forms in materials during thermo-mechanical processes, for example during production processes e.g. Rolling (metalworking), rolling. Consequently, the rolling process is often followed by a heat treatment to reduce the amount of unwanted texture. Controlling the production process in combination with the characterization of texture and the material's mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Several key characteristics of a superalloy are excellent mechanical strength, resistance to thermal creep deformation, good surface stability, and resistance to corrosion or oxidation. The crystal structure is typically face-centered cubic (FCC) austenitic. Examples of such alloys are Hastelloy, Inconel, Waspaloy, Rene alloys, Incoloy, MP98T, TMS alloys, and CMSX single crystal alloys. Superalloy development has relied heavily on both chemical and process innovations. Superalloys develop high temperature strength through solid solution strengthening and precipitation strengthening from secondary phase precipitates such as gamma prime and carbides. Oxidation or corrosion resistance is provided by elements such as aluminium and chromium. Superalloys are often cast as a single crystal—while grain boundaries may provide strength at low temperatures, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
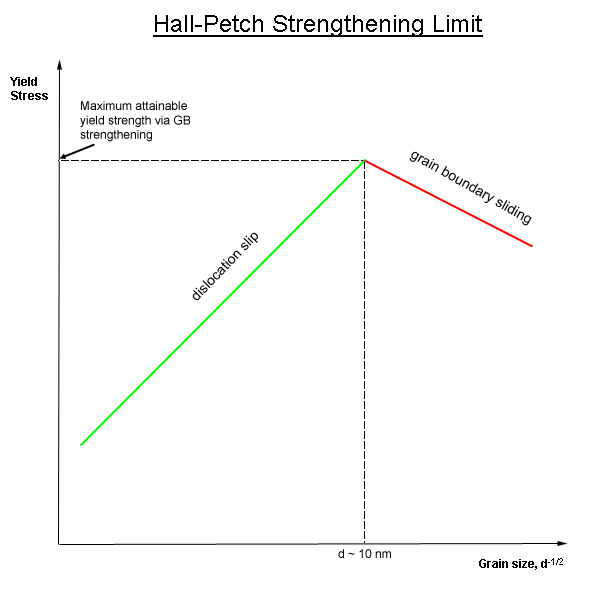
Grain Refinement
In materials science, grain-boundary strengthening (or Hall–Petch strengthening) is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable borders for dislocations and that the number of dislocations within a grain has an effect on how stress builds up in the adjacent grain, which will eventually activate dislocation sources and thus enabling deformation in the neighbouring grain as well. So, by changing grain size, one can influence the number of dislocations piled up at the grain boundary and yield strength. For example, heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.W.D. Callister. Fundamentals of Materials Science and Engineering, 2nd ed. Wiley & Sons. pp. 252. Theory In grain-boundary strengthening, the grain boundaries act as pinning points impeding further dislocation propagation. Since the lattice struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monocrystalline Silicon
Monocrystalline silicon, more often called single-crystal silicon, in short mono c-Si or mono-Si, is the base material for silicon-based discrete components and integrated circuits used in virtually all modern electronic equipment. Mono-Si also serves as a photovoltaic, light-absorbing material in the manufacture of solar cells. It consists of silicon in which the crystal lattice of the entire solid is continuous, unbroken to its edges, and free of any grain boundaries (i.e. a single crystal). Mono-Si can be prepared as an intrinsic semiconductor that consists only of exceedingly pure silicon, or it can be doped by the addition of other elements such as boron or phosphorus to make p-type or n-type silicon. Due to its semiconducting properties, single-crystal silicon is perhaps the most important technological material of the last few decades—the "silicon era", because its availability at an affordable cost has been essential for the development of the electronic devices on w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystalline Polycrystalline Amorphous
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macroscopic single crystals are usually identifiable by their geometrical shape, consisting of flat faces with specific, characteristic orientations. The scientific study of crystals and crystal formation is known as crystallography. The process of crystal formation via mechanisms of crystal growth is called crystallization or solidification. The word ''crystal'' derives from the Ancient Greek word (), meaning both "ice" and "rock crystal", from (), "icy cold, frost". Examples of large crystals include snowflakes, diamonds, and table salt. Most inorganic solids are not crystals but polycrystals, i.e. many microscopic crystals fused together into a single solid. Polycrystals include most metals, rocks, ceramics, and ice. A third category of sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grain Boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional crystallographic defect, defects in the crystal structure, and tend to decrease the electrical conductivity, electrical and thermal conductivity of the material. Most grain boundaries are preferred sites for the onset of corrosion and for the precipitation (chemistry), precipitation of new Phase (matter), phases from the solid. They are also important to many of the mechanisms of Creep (deformation), creep. On the other hand, grain boundaries disrupt the motion of dislocations through a material, so reducing crystallite size is a common way to improve mechanical strength, as described by the Hall–Petch relationship. High and low angle boundaries It is convenient to categorize grain boundaries according to the extent of misorientation between the two grains. ''Low-angle grain boundaries'' (''LAGB'') or ''subgrain boun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bronze Bell With Visible Material Structure
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such as arsenic or silicon. These additions produce a range of alloys that may be harder than copper alone, or have other useful properties, such as ultimate tensile strength, strength, ductility, or machinability. The three-age system, archaeological period in which bronze was the hardest metal in widespread use is known as the Bronze Age. The beginning of the Bronze Age in western Eurasia and India is conventionally dated to the mid-4th millennium BCE (~3500 BCE), and to the early 2nd millennium BCE in China; elsewhere it gradually spread across regions. The Bronze Age was followed by the Iron Age starting from about 1300 BCE and reaching most of Eurasia by about 500 BCE, although bronze continued to be much more widely used than it is in mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercooling
Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid or a gas below its melting point without it becoming a solid. It achieves this in the absence of a seed crystal or nucleus around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−55 °F). Droplets of supercooled water often exist in stratus and cumulus clouds. An aircraft flying through such a cloud sees an abrupt crystallization of these droplets, which can result in the formation of ice on the aircraft's wings or blockage of its instruments and probes. Animals utilize supercooling to survive in extreme temperatures. There are many mechanisms that aid in maintaining a liquid state, such as the production of antifreeze proteins, which bind to ice crystals to prevent water molecules from binding and spreading the growth of ice. The winter flounder is on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fracture
Fracture is the separation of an object or material into two or more pieces under the action of stress. The fracture of a solid usually occurs due to the development of certain displacement discontinuity surfaces within the solid. If a displacement develops perpendicular to the surface, it is called a normal tensile crack or simply a crack; if a displacement develops tangentially, it is called a shear crack, slip band or dislocation. Brittle fractures occur with no apparent deformation before fracture. Ductile fractures occur after visible deformation. Fracture strength, or breaking strength, is the stress when a specimen fails or fractures. The detailed understanding of how a fracture occurs and develops in materials is the object of fracture mechanics. Strength Fracture strength, also known as breaking strength, is the stress at which a specimen fails via fracture. This is usually determined for a given specimen by a tensile test, which charts the stress–strain cu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |