|
Pipecolic Acid
Pipecolic acid (piperidine-2-carboxylic acid) is an organic compound with the formula HNC5H9CO2H. It is a carboxylic acid derivative of piperidine and, as such, an amino acid, although not one encoded genetically. Like many other α-amino acids, pipecolic acid is chiral, although the S-stereoisomer is more common. It is a colorless solid. Its biosynthesis starts from lysine. CRYM, a taxon-specific protein that also binds thyroid hormones, is involved in the pipecolic acid pathway. Medicine It accumulates in pipecolic acidemia. Pipecolic acid can be associated with some forms of epilepsy. Occurrence and reactions Like most amino acids, pipecolic acid is a chelating agent. One complex is Cu(HNC5H9CO2)2(H2O)2. Pipecolic acid was identified in the Murchison meteorite. It also occurs in the leaves of the genus ''Myroxylon'', a tree from South America. See also * Bupivacaine * Efrapeptin Efrapeptins are peptides produced by fungi in the genus ''Tolypocladium'' that have antifunga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidine
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic compound, heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name ''Piper (genus), Piper'', which is the Latin word for Black pepper, pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring Solenopsin, solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson (chemist), Thomas Anderson and again, independently, in 1852 by the French chemist Auguste André Thomas Cahours, Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha And Beta Carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule. Numeric locants The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of numeric prefixes to indicate the position of substituents, generally by identifying the parent hydrocarbon chain and assigning the carbon atoms based on their substituents in order of precedence. For example, there are at least two isomers of the linear form of pentanone, a ketone that contains a chain of exactly five carbon atoms. There is an oxygen atom bonded to one of the middle three carbons (if it were bonded to an end carbon, the molecule would be an aldehyde, not a ketone), but it is not clear where it is located. In this example, the carbon atoms are numbered from one to five, which starts at one end and proceeds sequentially along the chain. Now the position of the oxygen atom can be defined as on carbon atom number two, three or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRYM
Mu-crystallin homolog also known as NADP-regulated thyroid-hormone-binding protein (THBP) is a protein that in humans is encoded by the ''CRYM'' gene. Multiple alternatively spliced transcript variants have been found for this gene. Function Crystallins are separated into two classes: taxon-specific and ubiquitous. The former class is also called phylogenetically-restricted crystallins. The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. This gene encodes a taxon-specific crystallin protein that binds NADPH and has sequence similarity to bacterial ornithine cyclodeaminases. The encoded protein does not perform a structural role in lens tissue, and instead it binds thyroid hormone for possible regulatory or developmental roles. Its enzyme function has been determined as a ketimine reductase, reducing cyclic ketimines to their reduced forms. Either NADH or NADPH can be used as cofactor. The mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
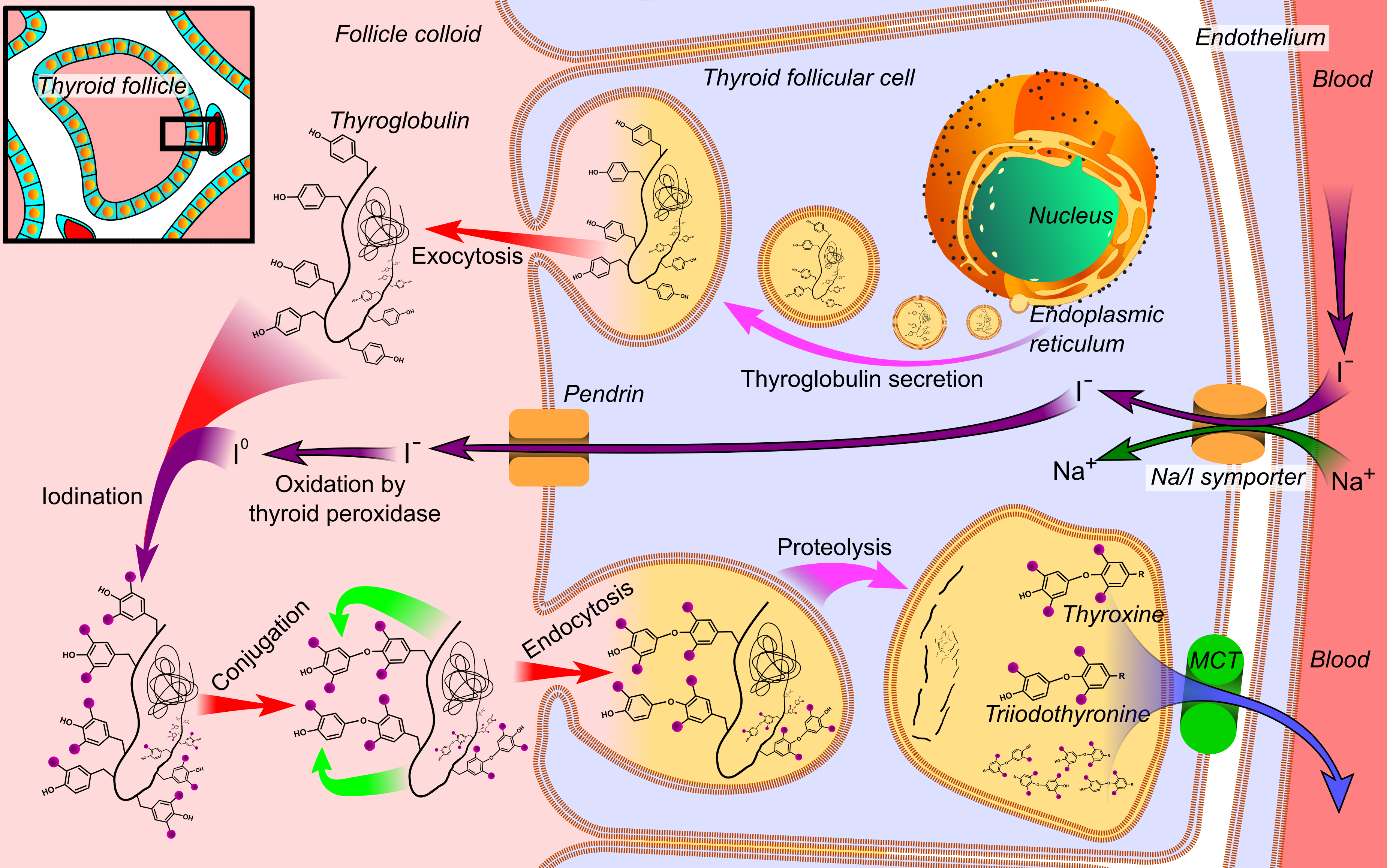
Thyroid Hormone
File:Thyroid_system.svg, upright=1.5, The thyroid system of the thyroid hormones T3 and T4 rect 376 268 820 433 Thyroid-stimulating hormone rect 411 200 849 266 Thyrotropin-releasing hormone rect 297 168 502 200 Hypothalamus rect 66 216 386 256 Anterior pituitary gland rect 66 332 342 374 Negative feedback rect 308 436 510 475 Thyroid gland rect 256 539 563 635 Thyroid hormones rect 357 827 569 856 Catecholamine rect 399 716 591 750 Metabolism desc bottom-left Thyroid hormones are any hormones produced and released by the thyroid gland, namely triiodothyronine (T3) and thyroxine (T4). They are tyrosine-based hormones that are primarily responsible for regulation of metabolism. T3 and T4 are partially composed of iodine. A deficiency of iodine leads to decreased production of T3 and T4, enlarges the thyroid tissue and will cause the disease known as simple goitre. The major form of thyroid hormone in the blood is thyroxine (T4), whose half-life of around one week is longer th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pipecolic Acidemia
Pipecolic acidemia is a very rare autosomal recessive metabolic disorder that is caused by a peroxisomal defect. Pipecolic acidemia can also be an associated component of Refsum disease with increased pipecolic acidemia (RDPA), as well as other peroxisomal disorders, including both infantile and adult Refsum disease, and Zellweger syndrome. The disorder is characterized by an increase in pipecolic acid levels in the blood, leading to neuropathy and hepatomegaly. See also * AASDHPPT * PHYH In enzymology, a phytanoyl-CoA dioxygenase () is an enzyme that catalyzes the chemical reaction :phytanoyl-CoA + 2-oxoglutarate + O2 \rightleftharpoons 2-hydroxyphytanoyl-CoA + succinate + CO2 The three substrates of this enzyme are phyta ... References External links Amino acid metabolism disorders Autosomal recessive disorders Rare diseases {{genetic-disorder-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epilepsy
Epilepsy is a group of non-communicable neurological disorders characterized by recurrent epileptic seizures. Epileptic seizures can vary from brief and nearly undetectable periods to long periods of vigorous shaking due to abnormal electrical activity in the brain. These episodes can result in physical injuries, either directly such as broken bones or through causing accidents. In epilepsy, seizures tend to recur and may have no immediate underlying cause. Isolated seizures that are provoked by a specific cause such as poisoning are not deemed to represent epilepsy. People with epilepsy may be treated differently in various areas of the world and experience varying degrees of social stigma due to the alarming nature of their symptoms. The underlying mechanism of epileptic seizures is excessive and abnormal neuronal activity in the cortex of the brain which can be observed in the electroencephalogram (EEG) of an individual. The reason this occurs in most cases of epilepsy is u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Murchison Meteorite
The Murchison meteorite is a meteorite that fell in Australia in 1969 near Murchison, Victoria. It belongs to the carbonaceous chondrite class, a group of meteorites rich in organic compounds. Due to its mass (over ) and the fact that it was an observed fall, the Murchison meteorite is one of the most studied of all meteorites. In January 2020, cosmochemists reported that the oldest material found on Earth to date are the silicon carbide particles from the Murchison meteorite, which have been determined to be 7 billion years old, about 2.5 billion years older than the 4.54-billion-year age of the Earth and the Solar System. The published study noted that "dust lifetime estimates mainly rely on sophisticated theoretical models. These models, however, focus on the more common small dust grains and are based on assumptions with large uncertainties." History On 28 September 1969 at approximately 10:58 a.m. local time, near Murchison, Victoria, in Australia, a bright ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myroxylon
''Myroxylon'' is a genus of Fabaceae native to Latin America. History The first described species in this genus was '' M. balsamum.'' It was originally described in 1753 by Linnaeus as ''Toluifera balsamum'', based on a specimen collected in the province of Cartagena (at the time Tolú was located in the province of Cartagena). The genus ''Myroxylon'' was first established by Linnaeus filius in 1781, when he described '' M. peruiferum'' based on a specimen collected by Mutis in South America. Although ''Toluifera'' is prior in term of publication time, ''Myroxylon'' is chosen as the conserved name and ''Toluifera'' is rejected. The name derives from Greek μύρρα (''myrrha'', "myrrh") and ξύλον (''xylon'', "wood"). Species Some authors recognize infra-specific taxa based, mainly, in their balsam phytochemistry; while other authors do not recognize such categories. There are reports of differences in composition of balsams obtained from ''M. balsamum'' var. ''balsamu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bupivacaine
Bupivacaine, marketed under the brand name Marcaine among others, is a medication used to decrease feeling in a specific area. In nerve blocks, it is injected around a nerve that supplies the area, or into the spinal canal's epidural space. It is available mixed with a small amount of epinephrine to increase the duration of its action. It typically begins working within 15 minutes and lasts for 2 to 8 hours. Possible side effects include sleepiness, muscle twitching, ringing in the ears, changes in vision, low blood pressure, and an irregular heart rate. Concerns exist that injecting it into a joint can cause problems with the cartilage. Concentrated bupivacaine is not recommended for epidural freezing. Epidural freezing may also increase the length of labor. It is a local anaesthetic of the amide group. Bupivacaine was discovered in 1957. It is on the World Health Organization's List of Essential Medicines. Bupivacaine is available as a generic medication. An implantable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Efrapeptin
Efrapeptins are peptides produced by fungi in the genus ''Tolypocladium'' that have antifungal, insecticidal, and mitochondrial ATPase inhibitory activities. They are produced via a biosynthetic pathway similar to but simpler than the Ciclosporin pathway, with nonribosomal peptide synthase (NRPS) and/or polyketide synthase (PKS) being the key elements. The amino acid sequences of efrapeptins are: :Efrapeptin F: Ac-Pip-Aib-Pip-Aib-Aib-Leu-bAla-Gly-Aib-Aib-Pip-Aib-Ala-Leu-Iva-Unk :Efrapeptin G: Ac-Pip-Aib-Pip-Iva-Aib-Leu-bAla-Gly-Aib-Aib-Pip-Aib-Ala-Leu-Iva-Unk ::Aib: 2-methylalanine; Iva: 2-ethylalanine; Unk: does not match to a known amino acid References External links * - Efrapeptin F Efrapeptin Fat ChemSpider ChemSpider is a database of chemicals. ChemSpider is owned by the Royal Society of Chemistry. Database The database contains information on more than 100 million molecules from over 270 data sources including: * EPA DSSTox * U.S. Food and D ... * - Efr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |