|
Perovskite (structure)
A perovskite is any material with a crystal structure following the formula ABX3, which was first discovered as the Perovskite, mineral called perovskite, which consists of calcium titanium oxide (CaTiO3). The mineral was first discovered in the Ural Mountains, Ural mountains of Russia by Gustav Rose in 1839 and named after Russian mineralogist L. A. Perovski (1792–1856). 'A' and 'B' are two positively charged ions (i.e. cations), often of very different sizes, and X is a negatively charged ion (an anion, frequently oxide) that bonds to both cations. The 'A' atoms are generally larger than the 'B' atoms. The ideal Cubic crystal system, cubic structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedron, cuboctahedral coordination. Additional perovskite forms may exist where either/both the A and B sites have a configuration of A1x-1A2x and/or B1y-1B2y and the X may deviate from the ideal coordination configu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns t ... as (XIIA2+VIB4+X2−3), known as the perovskite (structure), perovskite structure. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials. History The mineral was discovered in the Ural Mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist Lev Perovski (1792–1856). Perovskite's notable crystal structure was first described by Victor Goldschmidt in 1926 in his work on tolerance factors. The crystal structure was later published ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of the Earth's crust consists of oxides. Even materials considered pure elements often develop an oxide coating. For example, aluminium foil develops a thin skin of Al2O3 (called a passivation layer) that protects the foil from further corrosion.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Stoichiometry (the measurable relationship between reactants and chemical equations of a equation or reaction) Oxides are extraordinarily diverse in terms of stoichiometries and in terms of the structures of each stoichiometry. Most elements form oxides of more than one stoichiometry. A well known example is carbon monoxide and carbon dioxide.Greenwood, N. N.; & Earnshaw, A. (1997). Chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pulsed Laser Deposition
Pulsed laser deposition (PLD) is a physical vapor deposition (PVD) technique where a high-power pulsed laser beam is focused inside a vacuum chamber to strike a target of the material that is to be deposited. This material is vaporized from the target (in a plasma plume) which deposits it as a thin film on a substrate (such as a silicon wafer facing the target). This process can occur in ultra high vacuum or in the presence of a background gas, such as oxygen which is commonly used when depositing oxides to fully oxygenate the deposited films. While the basic setup is simple relative to many other deposition techniques, the physical phenomena of laser-target interaction and film growth are quite complex (see Process below). When the laser pulse is absorbed by the target, energy is first converted to electronic excitation and then into thermal, chemical and mechanical energy resulting in evaporation, ablation, plasma formation and even exfoliation.Pulsed Laser Deposition of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perovskite Oxide Thin Film
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as (XIIA2+VIB4+X2−3), known as the perovskite structure. Many different cations can be embedded in this structure, allowing the development of diverse engineered materials. History The mineral was discovered in the Ural Mountains of Russia by Gustav Rose in 1839 and is named after Russian mineralogist Lev Perovski (1792–1856). Perovskite's notable crystal structure was first described by Victor Goldschmidt in 1926 in his work on tolerance factors. The crystal structure was later published in 1945 from X-ray diffraction data on barium titanate by Helen Dick Megaw. Occurrence Found in the Earth's mantle, perovskite's occurrence at Khibina Massif is restricted to the silica under-saturated ultramafic rocks and foidolites, due to the instability in a paragenesis w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ruddlesden-Popper Phase
Ruddlesden-Popper (RP) phases are a type of perovskite structure that consists of two-dimensional perovskite-like slabs interleaved with cations. The general formula of an RP phase is ''An+1BnX3n+1'', where ''A'' and ''B'' are cations, ''X'' is an anion (e.g., oxygen), and ''n'' is the number of octahedral layers in the perovskite-like stack. Generally, it has a phase structure that results from the intergrowth of perovskite-type and NaCl-type (i.e., rocksalt-type) structures. These phases are named after S.N. Ruddlesden and P. Popper, who first synthesized and described a Ruddlesden-Popper structure in 1957. Crystal structure The general RP formula ''An+1BnX3n+1'' can be written ''An-1A’2BnX3n+1'', where ''A'' and ''A’'' are alkali, alkaline earth, or rare earth metals and ''B'' is a transition metal. The ''A'' cations are located in the perovskite layer and are 12-fold cuboctahedral coordinated by the anions (CN = 12). The ''A’'' cations have a coordination number of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aurivillius Phases
Aurivillius phases are a form of perovskite represented by the general formulae is ( Bi2 O2)(A''n''−1B''n''O3''n''+1) (where A is a large 12 co-ordinate cation, and B is a small 6 co-ordinate cation). Basically, their structure is built by alternating layers of i2O2sup>2+ and pseudo-perovskite blocks, with perovskite layers that are ''n'' octahedral layers in thickness. This crystal structure was first described in 1949 by Swedish chemist Bengt Aurivillius. The first interest in Aurivillius phases arose from the observation of ferroelectricity even for the simplest member, Bi2WO6 (n=1) of this crystallographic family. The Mo-homologous Aurivillius phase Bi2MoO6 was recently investigated as a potential LTCC material. Their oxide ion-conducting properties of Aurivillius phases were first discovered in the 1970s by Takahashi et al., and they have been used too for this purpose ever since. Aurivillius phase oxide materials are a class of lead-free ceramics. See also * Ruddlesd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferroelectricity
Ferroelectricity is a characteristic of certain materials that have a spontaneous electric polarization that can be reversed by the application of an external electric field. All ferroelectrics are also piezoelectric and pyroelectric, with the additional property that their natural electrical polarization is reversible. The term is used in analogy to ferromagnetism, in which a material exhibits a permanent magnetic moment. Ferromagnetism was already known when ferroelectricity was discovered in 1920 in Rochelle salt by Joseph Valasek.See and Thus, the prefix ''ferro'', meaning iron, was used to describe the property despite the fact that most ferroelectric materials do not contain iron. Materials that are both ferroelectric ''and'' ferromagnetic are known as multiferroics. Polarization When most materials are electrically polarized, the polarization induced, ''P'', is almost exactly proportional to the applied external electric field ''E''; so the polarization is a lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
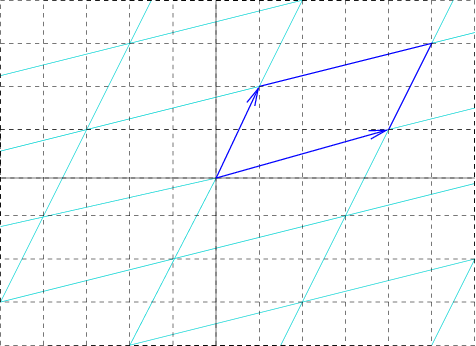
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium Titanate
Barium titanate (BTO) is an inorganic compound with chemical formula BaTiO3. Barium titanate appears white as a powder and is transparent when prepared as large crystals. It is a ferroelectric, pyroelectric, and piezoelectric ceramic material that exhibits the photorefractive effect. It is used in capacitors, electromechanical transducers and nonlinear optics. Structure The solid exists in one of four polymorphs depending on temperature. From high to low temperature, these crystal symmetries of the four polymorphs are cubic, tetragonal, orthorhombic and rhombohedral crystal structure. All of these phases exhibit the ferroelectric effect apart from the cubic phase. The high temperature cubic phase is easiest to describe, as it consists of regular corner-sharing octahedral TiO6 units that define a cube with O vertices and Ti-O-Ti edges. In the cubic phase, Ba2+ is located at the center of the cube, with a nominal coordination number of 12. Lower symmetry phases are stabili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium Titanate
Strontium titanate is an oxide of strontium and titanium with the chemical formula Sr Ti O3. At room temperature, it is a centrosymmetric paraelectric material with a perovskite structure. At low temperatures it approaches a ferroelectric phase transition with a very large dielectric constant ~104 but remains paraelectric down to the lowest temperatures measured as a result of quantum fluctuations, making it a quantum paraelectric. It was long thought to be a wholly artificial material, until 1982 when its natural counterpart—discovered in Siberia and named tausonite—was recognised by the IMA. Tausonite remains an extremely rare mineral in nature, occurring as very tiny crystals. Its most important application has been in its synthesized form wherein it is occasionally encountered as a diamond simulant, in precision optics, in varistors, and in advanced ceramics. The name ''tausonite'' was given in honour of Lev Vladimirovich Tauson (1917–1989), a Russian geochemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Titanate
Calcium titanate is an inorganic compound with the chemical formula Ca Ti O3. As a mineral, it is called perovskite, named after Russian mineralogist, L. A. Perovski (1792-1856). It is a colourless, diamagnetic solid, although the mineral is often coloured owing to impurities. Synthesis CaTiO3 can be prepared by the combination of CaO and TiO2 at temperatures >1300 °C. Sol-gel processes has been used to make a more pure substance, as well as lowering the synthesis temperature. These compounds synthesized are more compressible due to the powders from the sol-gel process as well and bring it closer to its calculated density (~4.04 g/ml). Structure Calcium titanate is obtained as orthorhombic crystals, more specifically perovskite structure. In this motif, the Ti(IV) centers are octahedral and the Ca2+ centers occupy a cage of 12 oxygen centres. Many useful materials adopt related structures, e.g. barium titanate or variations of the structure, e.g. yttrium barium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

_crystallographic_standard_alignment.png)