|
Para-Iodoamphetamine
''para''-Iodoamphetamine (PIA), also known as 4-iodoamphetamine (4-IA), is a research chemical of the phenethylamine and amphetamine chemical classes. It acts as a selective serotonin releasing agent and is also a MAOI Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especia .... PIA is rumored to be a serotonergic neurotoxin on the account of that being reported to be the case for ''para''-chloroamphetamine. However, PIA is a much weaker 5-HT neurotoxin than is the case for PCA. Analogs 5-IAI was an attempt to make a non-neurotoxic analog of PIA. See also * ''p''-Bromoamphetamine * ''p''-Chloroamphetamine * ''p''-Fluoroamphetamine * Iofetamine References Neurotoxins Substituted amphetamines Iodoarenes Serotonin-norepinephrine-dopamine releasing agents {{ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iofetamine
Iofetamine (iodine-123, 123I), brand names Perfusamine, SPECTamine), or ''N''-isopropyl-(123I)-''p''-iodoamphetamine (IMP), is a Lipid solubility, lipid-soluble amine and radiopharmaceutical drug used in neuroimaging, cerebral blood perfusion imaging with single-photon emission computed tomography (SPECT). Labeled with the radioactive isotope iodine-123, it is approved for use in the United States as a medical diagnosis, diagnostic aid in determining the localization of and in the evaluation of stroke, non-lacunar stroke and complex partial seizures, as well as in the early diagnosis of Alzheimer's disease. An structural analog, analogue of amphetamine, iofetamine has shown to inhibit the reuptake of serotonin and norepinephrine as well as induce the release of these neurotransmitters and of dopamine with similar potency (pharmacology), potencies to other substituted amphetamine, amphetamines like dextroamphetamine, d-amphetamine and para-chloroamphetamine, ''p''-chloroamphetamin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Fluoroamphetamine
4-Fluoroamphetamine (4-FA; 4-FMP; PAL-303; "Flux"), also known as ''para''-fluoroamphetamine (PFA) is a psychoactive research chemical of the phenethylamine and substituted amphetamine chemical classes. It produces stimulant and entactogenic effects. As a recreational drug, 4-FA is sometimes sold along with related compounds such as 2-fluoroamphetamine and 4-fluoromethamphetamine. Usage 4-FA is popular in the Netherlands where it is predominantly used for its specific effects (77% of users) rather than its legal status (18%). 4-FA has become illegal since May 2017. Effects The subjective effects of 4-fluoroamphetamine include euphoria which some find similar to the effects of MDMA and amphetamine, increased energy (stimulation), mood elevation, feelings of warmth and empathy, excessive talking, bruxism, and suppressed appetite (anorexic). The general course of effects involves primarily empathogenic effects for the first few hours, which fades out as increased stimulation d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Amphetamines
Substituted amphetamines are a class of compounds based upon the amphetamine structure; it includes all derivative compounds which are formed by replacing, or substituting, one or more hydrogen atoms in the amphetamine core structure with substituents. The compounds in this class span a variety of pharmacological subclasses, including stimulants, empathogens, and hallucinogens, among others. Examples of substituted amphetamines are amphetamine (itself), methamphetamine, ephedrine, cathinone, phentermine, mephentermine, bupropion, methoxyphenamine, selegiline, amfepramone (diethylpropion), pyrovalerone, MDMA (ecstasy), and DOM (STP). Some of amphetamine's substituted derivatives occur in nature, for example in the leaves of ''Ephedra'' and khat plants. Amphetamine was first produced at the end of the 19th century. By the 1930s, amphetamine and some of its derivative compounds found use as decongestants in the symptomatic treatment of colds and also occasionally as psychoac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Research Chemical
Research chemicals are chemical substances used by scientists for medical and scientific research purposes. One characteristic of a research chemical is that it is for laboratory research use only; a research chemical is not intended for human or veterinary use. This distinction is required on the labels of research chemicals, and is what exempts them from regulation under parts 100-740 in Title 21 of the Code of Federal Regulations ( 21CFR). Background Pharmacological research chemicals Research chemicals are fundamental in the development of novel pharmacotherapies. Common medical laboratory uses include ''in vivo'' and animal testing to determine therapeutic value, toxicology testing by contract research organizations to determine drug safety, and analysis by drug test and forensic toxicology labs for the purposes of evaluating human exposure. Many pharmacologically active chemicals are sold online under the guise of "research chemicals," when in reality they are untested d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation. Phenethylamine is sold as a dietary supplement for purported mood and weight loss-related therapeutic benefits; however, in orally ingested phenethylamine, a significant amount is metabolized in the small intestine by monoami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphetamine
Amphetamine (contracted from alpha- methylphenethylamine) is a strong central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. It is also commonly used as a recreational drug. Amphetamine was discovered in 1887 and exists as two enantiomers: levoamphetamine and dextroamphetamine. ''Amphetamine'' properly refers to a specific chemical, the racemic free base, which is equal parts of the two enantiomers in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an athletic performance enhancer and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant. It is a prescription drug in many countries, and unauthorized possession and distribution of amphetamine are often tightly controlled due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Class
Chemical classification systems attempt to classify elements or compounds according to certain chemical functional or structural properties. Whereas the structural properties are largely intrinsic, functional properties and the derived classifications depend to a certain degree on the type of chemical interaction partners on which the function is exerted. Sometimes other criteria like purely physical ones (e.g. molecular weight) or - on the other hand - functional properties above the chemical level are also used for building chemical taxonomies. Some systems mix the various levels, resulting in hierarchies where the domains are slightly confused, for example having structural and functional aspects end up on the same level. Whereas chemical function is closely dependent on chemical structure, the situation becomes more involved when e.g. pharmacological function is integrated, because the QSAR can usually not be directly computed from structural qualities. Physico-chemical cla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonin
Serotonin () or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex and multifaceted, modulating mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. Approximately 90% of the serotonin that the body produces is in the intestinal tract. Biochemically, the indoleamine molecule derives from the amino acid tryptophan, via the (rate-limiting) hydroxylation of the 5 position on the ring (forming the intermediate 5-hydroxytryptophan), and then decarboxylation to produce serotonin. Serotonin is primarily found in the enteric nervous system located in the gastrointestinal tract (GI tract). However, it is also produced in the central nervous system (CNS), specifically in the raphe nuclei located in the brainstem, Merkel cells located in the skin, pulmonary neuroendocrine cells and taste receptor cells in the tongue. Additionally, serotonin is stored in blood platelets and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Releasing Agent
A monoamine releasing agent (MRA), or simply monoamine releaser, is a drug that induces the synapse, release of a monoamine neurotransmitter from the synapse, presynaptic neuron into the synapse, leading to an increase in the extracellular concentrations of the neurotransmitter. Many drugs induce their effects in the body and/or brain via the release of monoamine neurotransmitters, e.g., trace amines, many substituted amphetamines, and related compounds. Types of MRAs MRAS can be classified by the monoamines they mainly release, although these drugs lie on a spectrum. * Selective for one neurotransmitter ** Serotonin releasing agent (SRA) ** Norepinephrine releasing agent (NRA) ** Dopamine releasing agent (DRA) * Non-selective, releasing two or more neurotransmitters ** Norepinephrine–dopamine releasing agent (NDRA) ** Serotonin–norepinephrine releasing agent (SNRA) ** Serotonin–dopamine releasing agent (SDRA) ** Serotonin–norepinephrine–dopamine releasing agent (SNDR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
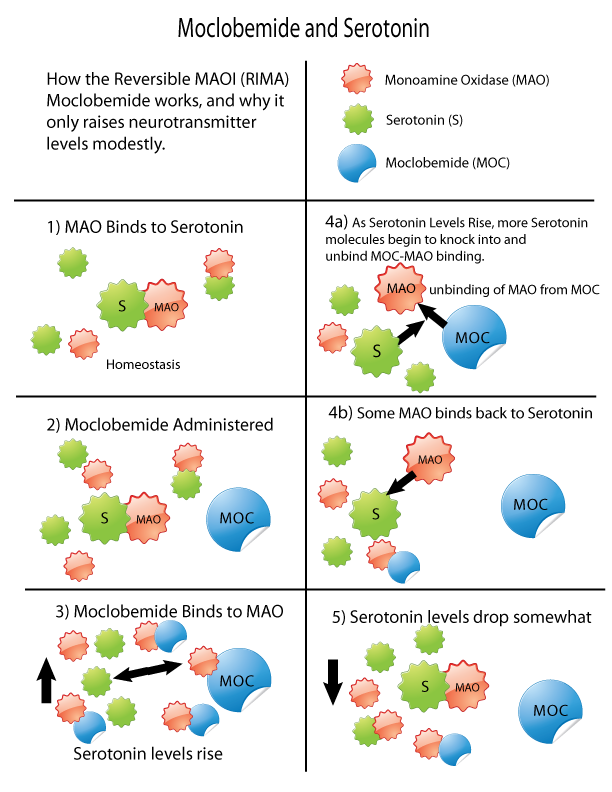
MAOI
Monoamine oxidase inhibitors (MAOIs) are a class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that selectively and reversibly inhibit the MAO-A enzyme. RIMAs are used clinically in the treatment of depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in the United States. Medical uses MAOIs have been found to be effective in the treatment of panic disorder with agoraphobia, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
5-IAI
5-Iodo-2-aminoindane (5-IAI) is a drug which acts as a releasing agent of serotonin, norepinephrine, and dopamine. It was developed in the 1990s by a team led by David E. Nichols at Purdue University. 5-IAI fully substitutes for MDMA in rodents and is a putative entactogen in humans. Unlike related aminoindane derivatives like MDAI and MMAI, 5-IAI causes some serotonergic neurotoxicity in rats, but is substantially less toxic than its corresponding amphetamine Amphetamine (contracted from alpha- methylphenethylamine) is a strong central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity. It is also commonly used ... homologue pIA, with the damage observed barely reaching statistical significance. Legal status Sweden's public health agency suggested classifying 5-IAI as a hazardous substance, on September 25, 2019. References {{DEFAULTSORT:Iodo-2-aminoindane, 5- 2-Aminoind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |