|
Organoarsenic Chemistry
Organoarsenic chemistry is the chemistry of compounds containing a chemical bond between arsenic and carbon. A few organoarsenic compounds, also called "organoarsenicals," are produced industrially with uses as insecticides, herbicides, and fungicides. In general these applications are declining in step with growing concerns about their impact on the environment and human health. The parent compounds are arsane and arsenic acid. Despite their toxicity, organoarsenic biomolecules are well known. History 140px, Cacodyl (tetramethyldiarsine) was one of the first organoarsenic compounds. Surprising for an area now considered of minor importance, organoarsenic chemistry played a prominent role in the history of the field of chemistry. The oldest known organoarsenic compound, the foul smelling cacodyl was reported in "cacodyl" (1760) and is sometimes classified as the first synthetic organometallic compound. The compound Salvarsan was one of the first pharmaceuticals, earning a Nobel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and ''halite'' reflects this correlation. All Group 1 metals form halides that are white solids at room temperature. A halide ion is a halogen atom bearing a negative charge. The halide anions are fluoride (), chloride (), bromide (), iodide () and astatide (). Such ions are present in all ionic halide salts. Halide minerals contain halides. All these halides are colourless, high melting crystalline solids having high negative enthalpies of formation. Test ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Hydroxy-3-nitrobenzenearsonic Acid
Roxarsone is an organoarsenic compound that has been used in poultry production as a feed additive to increase weight gain and improve feed efficiency, and as a coccidiostat. As of June 2011, it was approved for chicken feed in the United States, Canada, Australia, and 12 other countries. The drug was also approved in the United States and elsewhere for use in pigs. Its use in the United States was voluntarily ended by the manufacturers in June 2011 and has been illegal since 2013. Its use was immediately suspended in Malaysia. It was banned in Canada in August 2011. In Australia, its use in chicken feed was discontinued in 2012. Roxarsone has been banned in the European Union since 1999. Production and applications Roxarsone is a derivative of phenylarsonic acid (C6H5As(O)(OH)2). It was first reported in a 1923 British patent that described the nitration and diazotization of arsanilic acid. When blended with calcite powder, it is used in poultry feed premixes in some parts of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanearsonic Acid
Methylarsonic acid is an organoarsenic compound with the formula CH3AsO3H2. It is a colorless, water-soluble solid. Salts of this compound, e.g. disodium methyl arsonate, have been widely used in as herbicides and fungicides in growing cotton and rice. Reactions Near physiological pH, methanearsonic acid converts to its conjugate bases, the methylarsonates. These include CH3AsO3H− and . Synthesis and biosynthesis Reaction of arsenous acid with methyl iodide gives methylarsonic acid. This historically significant conversion is called the Meyer reaction: :As(OH)3 + CH3I + NaOH → CH3AsO(OH)2 + NaI + H2O The then-novel aspect of the reaction was that alkylation occurs at arsenic, leading to oxidation of arsenic from oxidation state +3 to +5. The biomethylation of arsenic compounds is thought to start with the formation of methanearsonates. Thus, trivalent arsenic compounds are methylated to give methanearsonate. ''S''-Adenosylmethionine is the methyl donor. The metha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bechamp Reaction
In organic synthesis the Béchamp reaction is used for producing arsonic acids from activated aromatic substrates. The reaction is an electrophilic aromatic substitution, using arsenic acid as the electrophile. The reaction proceeds according to this idealized stoichiometry for the preparation of arsanilic acid: : Reaction scope The reaction was first reported in 1863 by Antoine Béchamp. It is very analogous to the sulfonation of arenes. The Béchamp reaction was employed in the Nobel Prize The Nobel Prizes ( ; sv, Nobelpriset ; no, Nobelprisen ) are five separate prizes that, according to Alfred Nobel's will of 1895, are awarded to "those who, during the preceding year, have conferred the greatest benefit to humankind." Alfr ...-winning work on organoarsenicals by Paul Erlich. In one commercial application, the Béchamp reaction is reaction is used to produce roxarsone, which exhibits an anticoccidial action and promotes growth in animals. Further reading * R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine In organic chemistry, an aromatic amine is an organic compound consisting of an aromatic ring attached to an amine. It is a broad class of compounds that encompasses aniline Aniline is an organic compound with the formula C6 H5 NH2. Consi .... It is an industrially significant Commodity chemicals, commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It Combustion, ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archives Of Toxicology
''Archives of Toxicology'' is a peer-reviewed medical journal covering all aspects of toxicology. It was established in 1930 as ''Sammlung von Vergiftungsfällen'', renamed in 1954 into ''Archiv für Toxikologie'' and obtained its current title in 1974. The journal is published by Springer Science+Business Media and the editor-in-chief is Jan G. Hengstler ( Leibniz Research Centre for Working Environment and Human Factors). Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 5.153. References {{reflist External linksWebsite link Toxicology journals Monthly journals Publications established in 1930 Springer Science+Business M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
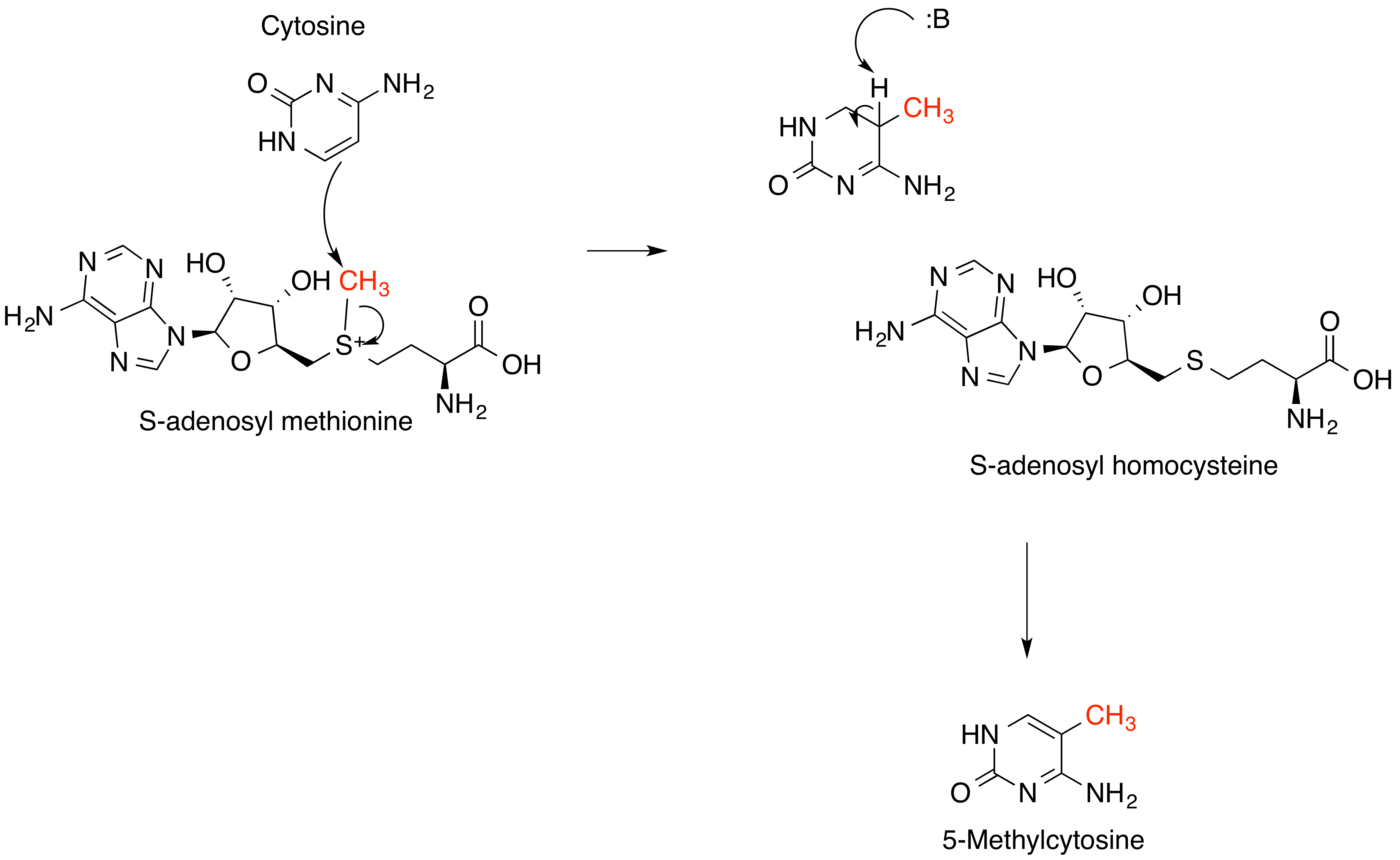
S-adenosylmethionine
''S''-Adenosyl methionine (SAM), also known under the commercial names of SAMe, SAM-e, or AdoMet, is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM is produced and consumed in the liver. More than 40 methyl transfers from SAM are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM was first discovered by Giulio Cantoni in 1952. In bacteria, SAM is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response; amino acid metabolism; transsulfuration; and more. In plants, SAM is crucial to the biosynthesis of ethylene, an important plant hormone and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanearsonate
Methylarsonic acid is an organoarsenic compound with the formula CH3AsO3H2. It is a colorless, water-soluble solid. Salts of this compound, e.g. disodium methyl arsonate, have been widely used in as herbicides and fungicides in growing cotton and rice. Reactions Near physiological pH, methanearsonic acid converts to its conjugate bases, the methylarsonates. These include CH3AsO3H− and . Synthesis and biosynthesis Reaction of arsenous acid with methyl iodide gives methylarsonic acid. This historically significant conversion is called the Meyer reaction: :As(OH)3 + CH3I + NaOH → CH3AsO(OH)2 + NaI + H2O The then-novel aspect of the reaction was that alkylation occurs at arsenic, leading to oxidation of arsenic from oxidation state +3 to +5. The biomethylation of arsenic compounds is thought to start with the formation of methanearsonates. Thus, trivalent arsenic compounds are methylated to give methanearsonate. ''S''-Adenosylmethionine is the methyl donor. The metha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biomethylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences. In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. In vitro methylation of tissue samples is also one method for reducing certain histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals, regulate gene expression, RNA processing and protein function. It has been recognized as a key process underlying epigenetics. Methanogenesis Methanogenesis, the proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylarsonic Acid
Phenylarsonic acid is the chemical compound with the formula C6H5AsO(OH)2, commonly abbreviated PhAsO3H2. This colourless solid is an organic derivative of arsenic acid, AsO(OH)3, where one OH group has been replaced by a phenyl group. The compound is a buffering agent and a precursor to other organoarsenic compounds, some of which are used in animal nutrition, e.g. 4-hydroxy-3-nitrobenzenearsonic acid. Preparation and structure PhAsO3H2 can be prepared in several routes, but a common one entails treatment of phenyl diazonium salts with sodium arsenite (prepared from arsenious acid and base) in the presence of a copper(II) catalyst. : + NaAsO3H2 → C6H5AsO3H2 + Na+ + N2 Related derivatives are prepared similarly. It was first prepared by Michaelis and Loenser. X-ray crystallography indicates that the molecules are connected by hydrogen-bonds consistent with short distance of 2.5 Å separating the oxygen atoms. The arsenic center is tetrahedral.Struchkov, Yu. T. "Cr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsonic Acids
Arsonic acids are a subset of organoarsenic compounds defined as oxyacids where a pentavalent arsenic atom is bonded to two hydroxyl groups, a third oxygen atom (this one with a double bond), and an organic substituent. The salts/conjugate bases of arsonic acids are called arsonates. Like all arsenic-containing compounds, arsonic acids are toxic and carcinogenic to humans. Arsonic acid refers to , the case where the substituent is a single hydrogen atom. The other arsonic acids can simply be viewed as hydrocarbyl derivatives of this base case. Arsenic acid results when the substituent is a hydroxyl group. Methylarsonic acid results when the substituent is a methyl group. Phenylarsonic acid results when the substituent is a phenyl group. Syntheses The Béchamp reaction is used to produce arsonic acids from activated aromatic substrates. The reaction is an electrophilic aromatic substitution, using arsenic acid as the electrophile. The reaction proceeds according to this idealiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |