|
Organoaluminium
Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins. History The first organoaluminium compound (C2H5)3Al2I3 was discovered in 1859. Organoaluminium compounds were, however, little known until the 1950s when Karl Ziegler and colleagues discovered the direct synthesis of trialkylaluminium compounds and applied these compounds to catalytic olefin polymerization. This line of research ultimately resulted in the Nobel Prize to Ziegler. Structure and bonding Aluminium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triethylaluminium
Triethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( C2H5)6 (abbreviated as Al2Et6 or TEA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to trimethylaluminium. Structure and bonding The structure and bonding in Al2R6 and diborane are analogous (R = alkyl). Referring to Al2Me6, the Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively. The Al center is tetrahedral. The carbon atoms of the bridging ethyl groups are each surrounded by five neighbors: carbon, two hydrogen atoms and two aluminium atoms. The ethyl groups interchange readily intramolecularly. At higher temperatures, the dimer cracks into monomeric AlEt3. Synthesis and reactions Triethylaluminium can be formed via several routes. The discovery of an efficient route was a significant technological achievement. The multistep process uses aluminium m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylaluminium Sesquichloride
Ethylaluminium sesquichloride, also called EASC, is an industrially important organoaluminium compound used primarily as a precursor to triethylaluminium and as a catalyst component in Ziegler–Natta type systems for olefin and diene polymerizations. Other applications include use in alkylation reactions and as a catalyst component in linear oligomerization and cyclization of unsaturated hydrocarbons. EASC is a colourless liquid, spontaneously combustible in air and reacts violently when in contact with water and many other compounds.Aluminum alkyls. Albemarle Corporation 2010 Production Methyl, ethyl, and other alkyl or aralkyl halides that are not dehydrohalogenated readily can react with aluminium metal in an exothermic process to form organoalu ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethylaluminium
Trimethylaluminium is one of the simplest examples of an organoaluminium compound. Despite its name it has the formula Al2( CH3)6 (abbreviated as Al2Me6 or TMA), as it exists as a dimer. This colorless liquid is pyrophoric. It is an industrially important compound, closely related to triethylaluminium. Structure and bonding The structure and bonding in Al2R6 and diborane are analogous (R = alkyl). In Al2Me6, the Al-C(terminal) and Al-C(bridging) distances are 1.97 and 2.14 Å, respectively. The Al center is tetrahedral. The carbon atoms of the bridging methyl groups are each surrounded by five neighbors: three hydrogen atoms and two aluminium atoms. The methyl groups interchange readily intramolecularly. At higher temperatures, the dimer cracks into monomeric AlMe3. Synthesis TMA is prepared via a two-step process that can be summarized as follows: :2 Al + 6 CH3Cl + 6 Na → Al2(CH3)6 + 6 NaCl Applications Catalysis Starting with the invention of Ziegler-Natta catalysis, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triisobutylaluminium
Triisobutylaluminium (TiBA) is an organoaluminium compound with the formula Al(CH2CH(CH3)2)3. This colorless pyrophoricity, pyrophoric liquid is mainly used to make linear primary alcohol (chemistry), alcohols and α-olefins.Michael J. Krause, Frank Orlandi, Alfred T. Saurage, Joseph R. Zietz Jr. "Aluminum Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. Structure Triisobutylaluminium exists in equilibrium with its dimer (chemistry), dimer. The equilibrium constant, KD, is 3.810 at 20 °C. : 2Al(CH2CH(CH3)2)3 [Al(CH2CH(CH3)2)3]2 In the dimer, the bridging carbon-aluminium bond is elongated and exhibits evidence of restricted rotation. For the sake of simplicity, TiBA is written as the monomer in this article. Synthesis Trialkylaluminium compounds are available industrially through the reactions of aluminium powder, hydrogen gas, and the desired alkenes. The synthesis of TiBA requires two steps; the first step produces diisob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisobutylaluminium Hydride
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (''i''-Bu2AlH)2, where ''i''-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis. Properties Like most organoaluminum compounds, the compound's structure is most probably more than that suggested by its empirical formula. A variety of techniques, not including X-ray crystallography, suggest that the compound exists as a dimer and a trimer, consisting of tetrahedral aluminium centers sharing bridging hydride ligands. Hydrides are small and, for aluminium derivatives, are highly basic, thus they bridge in preference to the alkyl groups. DIBAL can be prepared by heating triisobutylaluminium (itself a dimer) to induce beta-hydride elimination: :(''i''-Bu3Al)2 → (''i''-Bu2AlH)2 + 2 (CH3)2C=CH2 Although DIBAL can be purchased commercially as a colorless liquid, it is more commonly purchased and dispensed as a solution in an organic sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyolefin
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds ( polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylcyclopentadiene
1,2,3,4,5-Pentamethylcyclopentadiene is a cyclic diene with the formula C5Me5H (Me = CH3). 1,2,3,4,5-Pentamethylcyclopentadiene is the precursor to the ligand ''1,2,3,4,5-pentamethylcyclopentadienyl'', which is often denoted Cp* (C5Me5) and read as "C P star", the "star" signifying the five methyl groups radiating from the core of the ligand. In contrast to less-substituted cyclopentadiene derivatives, Cp*H is not prone to dimerization. Synthesis Pentamethylcyclopentadiene is commercially available. It was first prepared from tiglaldehyde via 2,3,4,5-tetramethylcyclopent-2-enone. Alternatively, 2-butenyllithium adds to ethyl acetate followed by acid-catalyzed dehydrocyclization: Organometallic derivatives Cp*H is a precursor to organometallic compounds containing the ligand, commonly called Cp*−. Some representative reactions leading to such Cp*–metal complexes follow: :Cp*H + C4H9Li → Cp*Li + C4H10 :Cp*Li + TiCl4 → Cp*TiCl3 + LiCl Some Cp* co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton NMR
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the structure of its molecules. In samples where natural hydrogen (H) is used, practically all the hydrogen consists of the isotope 1H (hydrogen-1; i.e. having a proton for a nucleus). Simple NMR spectra are recorded in solution, and solvent protons must not be allowed to interfere. Deuterated (deuterium = 2H, often symbolized as D) solvents especially for use in NMR are preferred, e.g. deuterated water, D2O, deuterated acetone, (CD3)2CO, deuterated methanol, CD3OD, deuterated dimethyl sulfoxide, (CD3)2SO, and deuterated chloroform, CDCl3. However, a solvent without hydrogen, such as carbon tetrachloride, CCl4 or carbon disulfide, CS2, may also be used. Historically, deuterated solvents were supplied with a small amount (typically 0.1%) of tet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octet Rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens; although more generally the rule is applicable for the s-block and p-block of the periodic table. Other rules exist for other elements, such as the duplet rule for hydrogen and helium, or the 18-electron rule for transition metals. The valence electrons can be counted using a Lewis electron dot diagram as shown at the right for carbon dioxide. The electrons shared by the two atoms in a covalent bond are counted twice, once for each atom. In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in both the carbon octet and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
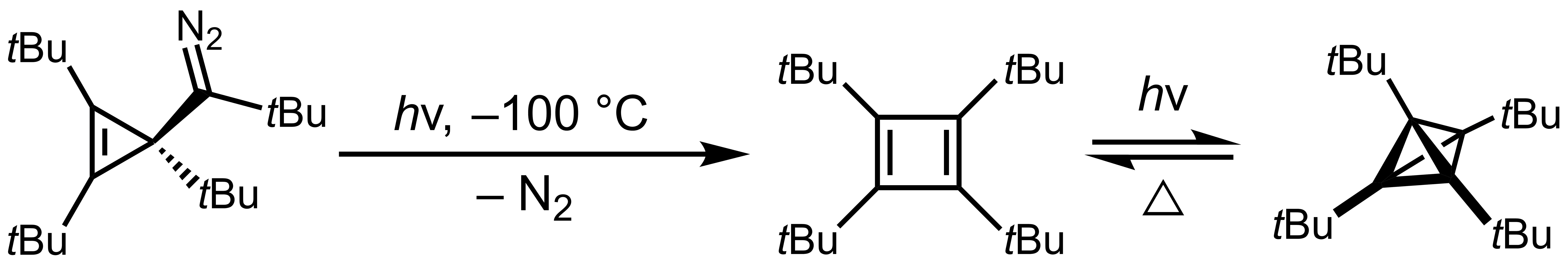
Tetrahedrane
Tetrahedrane is a hypothetical platonic hydrocarbon with chemical formula and a tetrahedral structure. The molecule would be subject to considerable angle strain and has not been synthesized as of 2021. However, a number of derivatives have been prepared. In a more general sense, the term ''tetrahedranes'' is used to describe a class of molecules and ions with related structure, e.g. white phosphorus. Organic tetrahedranes In 1978, Günther Maier prepared tetra- ''tert''-butyl-tetrahedrane. These bulky substituents envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain. Tetrahedrane is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo .1.0.02,4utane. Unsubstituted tetrahedrane () remains elusive, although it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |