|
Organotantalum Chemistry
Organotantalum chemistry is the chemistry of chemical compounds containing a carbon-to-tantalum chemical bond. A wide variety of compound have been reported, initially with cyclopentadienyl and CO ligands. Oxidation states vary from Ta(V) to Ta(-I). Classes of organotantalum compounds Alkyl and aryl complexes Pentamethyltantalum was reported by Richard Schrock in 1974. Salts of a(CH3)6sup>− are prepared by alkylation of TaF5 using methyl lithium: :TaF5 + 6 LiCH3 → Li a(CH3)6+ 5 LiF Alkylidene complexes Tantalum alkylidene complexes arise by treating trialkyltantalum dichloride with alkyl lithium reagents. This reaction initially forms a thermally unstable tetraalkyl-monochloro-tantalum complex, which undergoes α-hydrogen elimination, followed by alkylation of the remaining chloride. Tantalum alkylidene complexes are nucleophilic. They effect a number of reactions including: olefinations, olefin metathesis, hydroaminoalkylation of olefins, and conju ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olefin Metathesis
Olefin metathesis is an organic reaction that entails the redistribution of fragments of alkenes (olefins) by the scission and regeneration of carbon-carbon double bonds. Because of the relative simplicity of olefin metathesis, it often creates fewer undesired by-products and hazardous wastes than alternative organic reactions. For their elucidation of the reaction mechanism and their discovery of a variety of highly active catalysts, Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock were collectively awarded the 2005 Nobel Prize in Chemistry. Catalysts The reaction requires metal catalysts. Most commercially important processes employ heterogeneous catalysts. The heterogeneous catalysts are often prepared by in-situ activation of a metal halides (MClx) using organoaluminium or organotin compounds, e.g. combining MClx–EtAlCl2. A typical catalyst support is alumina. Commercial catalysts are often based on molybdenum and ruthenium. Well-defined organometallic co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne Complexes 1 Allylic Alcohol
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclotrimerization
In organic chemistry, an alkyne trimerisation is a +2+2nbsp;cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthesis. Being a cycloaddition reaction, it has high atom economy. Many variations have been developed, including cyclisation of mixtures of alkynes and alkenes as well as alkynes and nitriles. Mechanism and stereochemistry Trimerisation of acetylene to benzene is highly exergonic, proceeding with a free energy change of 142 kcal/mol at room temperature. Kinetic barriers however prevent the reaction from proceeding smoothly. The breakthrough came in 1948, when Reppe and Schweckendiek reported their wartime results showing that nickel compounds are effective catalysts: : 3 RC2H -> C6R3H3 Since this discovery, many other cyclotrimerisations have been reported. Mechanism In terms of mechanism, the reactions begi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Trichloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms hexahydrate with the formula , containing six water molecules of hydration. Both are colourless crystals, but samples are often contaminated with iron(III) chloride, giving a yellow color. The anhydrous material is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. The compound is often cited as a Lewis acid. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature. Structure Anhydrous adopts three structures, depending on the temperature and the state (solid, liquid, gas). Solid has a sheet-like layered structure with cubic close-packed chloride ions. In this framework, the Al centres exhibit octahedral coordination geometry. In contrast, has a more mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
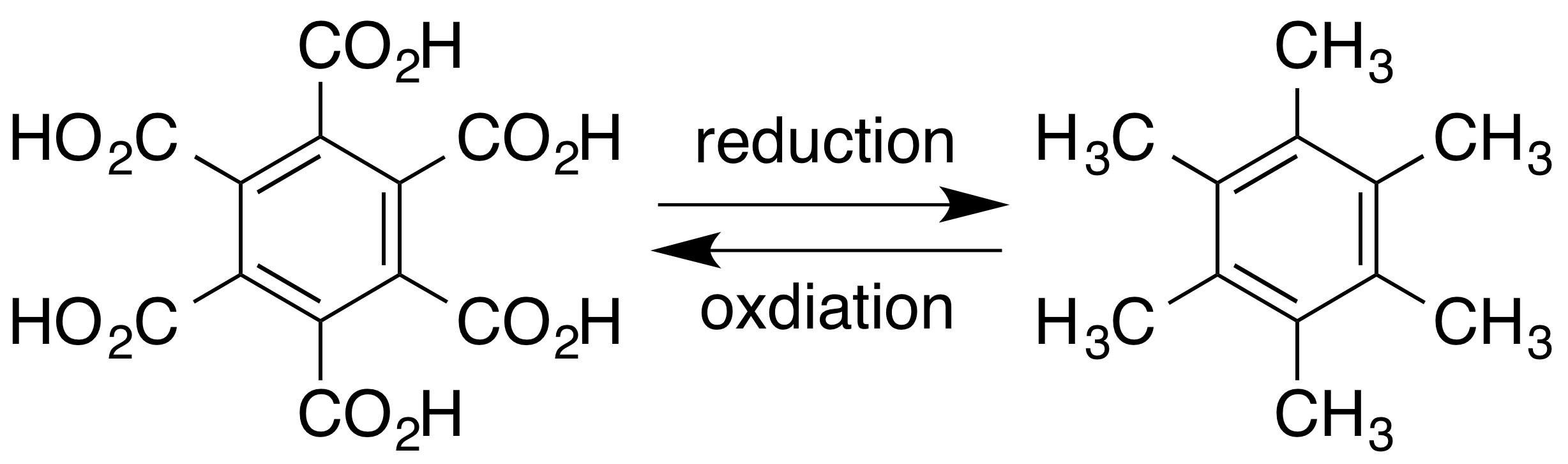
Hexamethylbenzene
Hexamethylbenzene, also known as mellitene, is a hydrocarbon with the molecular formula C12H18 and the condensed structural formula C6(CH3)6. It is an aromatic compound and a derivative of benzene, where benzene's six hydrogen atoms have each been replaced by a methyl group. In 1929 Kathleen Lonsdale reported the crystal structure of hexamethylbenzene, demonstrating that the central ring is hexagonal and flat and thereby ending an ongoing debate about the physical parameters of the benzene system. This was a historically significant result, both for the field of X-ray crystallography and for understanding aromaticity. The compound can be prepared by reacting phenol with methanol at elevated temperatures over a suitable solid catalyst such as alumina. The mechanism of the process has been studied extensively, with several intermediates having been identified. Alkyne trimerisation of dimethylacetylene also yields hexamethylbenzene in the presence of a suitable catalyst. Hexamet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocyanide
An isocyanide (also called isonitrile or carbylamine) is an organic compound with the functional group –. It is the isomer of the related nitrile (–C≡N), hence the prefix is ''isocyano''.IUPAC Goldboo''isocyanides''/ref> The organic fragment is connected to the isocyanide group through the nitrogen atom, not via the carbon. They are used as building blocks for the synthesis of other compounds. Properties Structure and bonding The C-N distance in isocyanides is 115.8 pm in methyl isocyanide. The C-N-C angles are near 180°. Akin to carbon monoxide, isocyanides are described by two resonance structures, one with a triple bond between the nitrogen and the carbon and one with a double bond between. The π lone pair of the nitrogen stabilizes the structure and is responsible of the linearity of isocyanides, although the reactivity of isocyanides reflects some carbene character, at least in a formal sense. Thus, both resonance structures are useful representations. They are sus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arene
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping of molecules based on smell, before their general chemical properties are understood. The current definition of aromatic compounds does not have any relation with their smell. Heteroarenes are closely related, since at least one carbon atom of CH group is replaced by one of the heteroatoms oxygen, nitrogen, or sulfur. Examples of non-benzene compounds with aromatic properties are furan, a heterocyclic compound with a five-membered ring that includes a single oxygen atom, and pyridine, a heterocyclic compound with a six-membered ring containing one nitrogen atom. Hydrocarbons without an aromatic ring are called aliphatic. Benzene ring model Benzene, C6H6, is the least complex aromatic hydrocarbon, and it was the first one named as such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylcyclopentadiene
1,2,3,4,5-Pentamethylcyclopentadiene is a cyclic diene with the formula C5Me5H (Me = CH3). 1,2,3,4,5-Pentamethylcyclopentadiene is the precursor to the ligand ''1,2,3,4,5-pentamethylcyclopentadienyl'', which is often denoted Cp* (C5Me5) and read as "C P star", the "star" signifying the five methyl groups radiating from the core of the ligand. In contrast to less-substituted cyclopentadiene derivatives, Cp*H is not prone to dimerization. Synthesis Pentamethylcyclopentadiene is commercially available. It was first prepared from tiglaldehyde via 2,3,4,5-tetramethylcyclopent-2-enone. Alternatively, 2-butenyllithium adds to ethyl acetate followed by acid-catalyzed dehydrocyclization: Organometallic derivatives Cp*H is a precursor to organometallic compounds containing the ligand, commonly called Cp*−. Some representative reactions leading to such Cp*–metal complexes follow: :Cp*H + C4H9Li → Cp*Li + C4H10 :Cp*Li + TiCl4 → Cp*TiCl3 + LiCl Some Cp* co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |