|
Nitronic Acid
A nitronate (IUPAC: azinate) in organic chemistry is a functional group with the general structure . It is the anion of nitronic acid (sometimes also called an aci, or an azinic acid), a tautomeric form of a nitro compound. Just as ketones and aldehydes can exist in equilibrium with their enol tautomer, nitro compounds exist in equilibrium with their nitronate tautomer under basic conditions. In practice they are formed by the deprotonation of the α-carbon, the pka of which is typically around 17. : Nitronates are formed as intermediates in the Henry reaction and Nef reaction In organic chemistry, the Nef reaction is an organic reaction describing the acid hydrolysis of a salt of a primary or secondary nitroalkane () to an aldehyde () or a ketone () and nitrous oxide (). The reaction has been the subject of several ..., the latter of which also demonstrates the instability of the nitronic acid form. The nitronate has two different resonance structures, one with a negative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitronate
A nitronate (IUPAC: azinate) in organic chemistry is a functional group with the general structure . It is the anion of nitronic acid (sometimes also called an aci, or an azinic acid), a tautomeric form of a nitro compound. Just as ketones and aldehydes can exist in equilibrium with their enol tautomer, nitro compounds exist in equilibrium with their nitronate tautomer under basic conditions. In practice they are formed by the deprotonation of the α-carbon, the pka of which is typically around 17. : Nitronates are formed as intermediates in the Henry reaction and Nef reaction In organic chemistry, the Nef reaction is an organic reaction describing the acid hydrolysis of a salt of a primary or secondary nitroalkane () to an aldehyde () or a ketone () and nitrous oxide (). The reaction has been the subject of sever ..., the latter of which also demonstrates the instability of the nitronic acid form. The nitronate has two different resonance structures, one with a negative ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Lynn Soby. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomer
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is best described as the "average" of the idealized, hypothe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitro Compound
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitro group is also strongly electron-withdrawing. Because of this property, bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid. Synthesis Preparation of aromatic nitro compounds Aromatic nitro compounds are typically synthesized by nitration. Nitration is achieved using a mixture of nitric acid and sulfuric acid, which produce the nitronium ion (), which is the electrophile: + The nitration product produced on the la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroethane Deprotonation
Nitroethane is an organic compound having the chemical formula C2H5NO2. Similar in many regards to nitromethane, nitroethane is an oily liquid at standard temperature and pressure. Pure nitroethane is colorless and has a fruity odor. Preparation Nitroethane is produced industrially by treating propane with nitric acid at 350–450 °C. This exothermic reaction produces four industrially significant nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane. The reaction involves free radicals, such as CH3CH2CH2O., which arise via homolysis of the corresponding nitrite ester. These alkoxy radicals are susceptible to C—C fragmentation reactions, which explains the formation of a mixture of products.Sheldon B. Markofsky “Nitro Compounds, Aliphatic” in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2002. . Alternatively, nitroethane can be produced by the Victor Meyer reaction of haloethanes such as chloroethane, bromoethane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
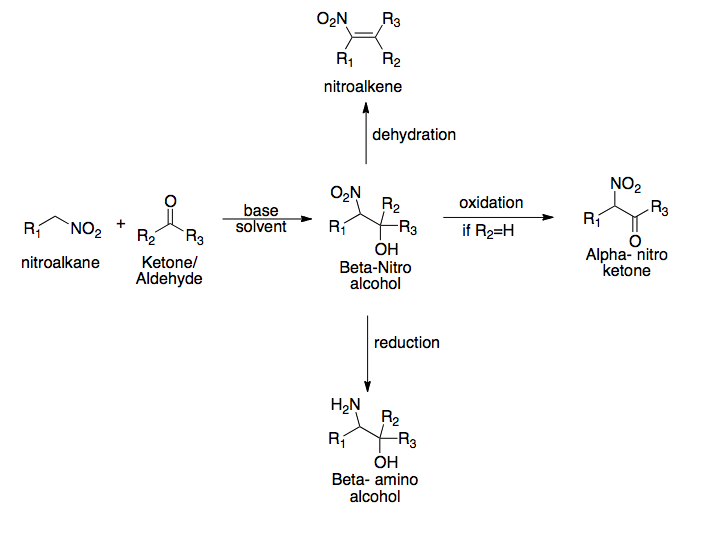
Henry Reaction
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction (nitroalkane, aldehyde, and alcohol). It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols" (aldehyde and alcohol). The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nef Reaction
In organic chemistry, the Nef reaction is an organic reaction describing the acid hydrolysis of a salt of a primary or secondary nitroalkane () to an aldehyde () or a ketone () and nitrous oxide (). The reaction has been the subject of several literature reviews. The reaction was reported in 1894 by the chemist John Ulric Nef, who treated the sodium salt of nitroethane with sulfuric acid resulting in an 85–89% yield of nitrous oxide and at least 70% yield of acetaldehyde. However, the reaction was pioneered a year earlier in 1893 by Konovalov, who converted the potassium salt of 1-phenylnitroethane with sulfuric acid to acetophenone. Reaction mechanism The reaction mechanism starting from the nitronate salt as the resonance structures 1a and 1b is depicted below: The salt is protonated forming the nitronic acid 2 (in some cases these nitronates have been isolated) and once more to the iminium ion 3. This intermediate is attacked by water in a nucleophilic addition for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |