|
Nitroethane
Nitroethane is an organic compound having the chemical formula C2H5NO2. Similar in many regards to nitromethane, nitroethane is an oily liquid at standard temperature and pressure. Pure nitroethane is colorless and has a fruity odor. Preparation Nitroethane is produced industrially by treating propane with nitric acid at 350–450 °C. This exothermic reaction produces four industrially significant nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane. The reaction involves free radicals, such as CH3CH2CH2O., which arise via homolysis of the corresponding nitrite ester. These alkoxy radicals are susceptible to C—C fragmentation reactions, which explains the formation of a mixture of products.Sheldon B. Markofsky “Nitro Compounds, Aliphatic” in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2002. . Alternatively, nitroethane can be produced by the Victor Meyer reaction of haloethanes such as chloroethane, bromo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl-2-nitropropene
1-Phenyl-2-nitropropene, or simply phenyl-2-nitropropene, or P2NP, as it is commonly referred to, is a chemical compound from the aromatic group of compounds, with the formula C9H9NO2. It is a light-yellow crystalline solid with a distinct smell. Phenyl-2-nitropropene is used in the pharmaceutical industry to manufacture the drug Adderall, an amphetamine mixture used to treat ADHD and narcolepsy. P2NP and other similar nitrostyrenes are also employed in the clandestine manufacture of drugs of the amphetamine class, and are listed as drug precursors in many countries. Uses In the pharmaceutical industry, P2NP is used to produce a racemic amphetamine mixture, branded under the trade names Adderall and Mydayis, amongst others. In this case, the double bond is hydrogenated and the nitro group is reduced, thus 1-phenyl-2-nitropropene becomes 1-phenyl-2-aminopropane, which is another name for amphetamine. Different reducing agents and solvents are used for this reaction, and various pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitromethane
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula . It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft. Preparation Nitromethane is produced industrially by combining propane and nitric acid in the gas phase at 350–450 °C (662–842 °F). This exothermic reaction produces the four industrially significant nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane. The reaction involves free radicals, including t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitromethane
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula . It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft. Preparation Nitromethane is produced industrially by combining propane and nitric acid in the gas phase at 350–450 °C (662–842 °F). This exothermic reaction produces the four industrially significant nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane. The reaction involves free radicals, including t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-nitropropane
1-Nitropropane (1-NP) is a solvent. It is a colorless liquid, an isomer of 2-nitropropane (2-NP), and classified as a nitro compound. Preparation 1-nitropropane is produced industrially by the reaction of propane and nitric acid. This reaction forms four nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane. 1-nitropropane is also a byproduct of the process for making 2-nitropropane, which is done by vapour phase nitration of propane. Uses Most 1-nitropropane is used as a starting material for other compounds. The other uses are solvent-based paints, solvent-based inks and adhesives, and as a solvent for chemical reactions. Safety 1-nitropropane is toxic to humans and can cause damage to the kidneys and liver. The vapours are irritating for the lungs and eyes and the maximum exposure rate is 25 ppm. It is not known to be a carcinogen. Reactions 1-nitropropane decomposes under the influence of heat into toxic gases. It also reacts violently with oxidizin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitro Compounds
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of the most common explosophores (functional group that makes a compound explosive) used globally. The nitro group is also strongly electron-withdrawing. Because of this property, bonds alpha (adjacent) to the nitro group can be acidic. For similar reasons, the presence of nitro groups in aromatic compounds retards electrophilic aromatic substitution but facilitates nucleophilic aromatic substitution. Nitro groups are rarely found in nature. They are almost invariably produced by nitration reactions starting with nitric acid. Synthesis Preparation of aromatic nitro compounds Aromatic nitro compounds are typically synthesized by nitration. Nitration is achieved using a mixture of nitric acid and sulfuric acid, which produce the nitronium ion (), which is the electrophile: + The nitration product produced on the la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Nitrate
Ethyl nitrate is the ethyl ester of nitric acid and has the chemical formula . It is a colourless, volatile, explosive, and highly flammable liquid. It is used in organic synthesis and as an intermediate in the preparation of some drugs, dyes, and perfumes. Ethyl nitrate is found in the atmosphere, where it can react with other gases to form smog. Originally thought to be a pollutant, formed mainly by the combustion of fossil fuels, recent analysis of ocean water samples reveal that in places where cool water rises from the deep, the water is saturated with alkyl nitrates, likely formed by natural processes. Preparation Ethyl nitrate has been prepared by bubbling gaseous nitryl fluoride through ethanol Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl ... at −10 °C. The re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MilliPascal Second
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI), and is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is defined as one newton per square metre and is equivalent to 10 barye (Ba) in the CGS system. The unit of measurement called standard atmosphere (atm) is defined as 101,325 Pa. Common multiple units of the pascal are the hectopascal (1 hPa = 100 Pa), which is equal to one millibar, and the kilopascal (1 kPa = 1000 Pa), which is equal to one centibar. Meteorological observations typically report atmospheric pressure in hectopascals per the recommendation of the World Meteorological Organization, thus a standard atmosphere (atm) or typical sea-level air pressure is about 1013 hPa. Reports in the United States typically use inches of mercury or millibars (hectopascals). In Canada these reports are given in kilopascals. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
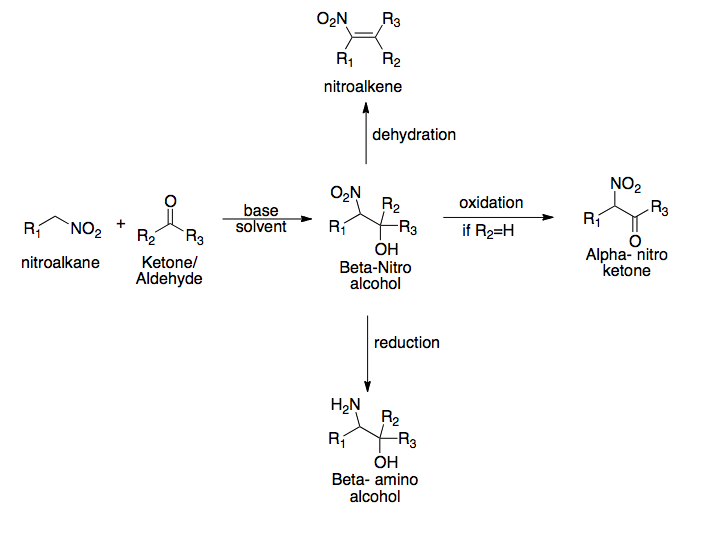
Henry Reaction
The Henry reaction is a classic carbon–carbon bond formation reaction in organic chemistry. Discovered in 1895 by the Belgian chemist Louis Henry (1834–1913), it is the combination of a nitroalkane and an aldehyde or ketone in the presence of a base to form β-nitro alcohols. This type of reaction is also referred to as a nitroaldol reaction (nitroalkane, aldehyde, and alcohol). It is nearly analogous to the aldol reaction that had been discovered 23 years prior that couples two carbonyl compounds to form β-hydroxy carbonyl compounds known as "aldols" (aldehyde and alcohol). The Henry reaction is a useful technique in the area of organic chemistry due to the synthetic utility of its corresponding products, as they can be easily converted to other useful synthetic intermediates. These conversions include subsequent dehydration to yield nitroalkenes, oxidation of the secondary alcohol to yield α-nitro ketones, or reduction of the nitro group to yield β-amino alcohols. Many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,4-dimethoxybenzaldehyde
Veratraldehyde (3,4-dimethoxybenzaldehyde) is an organic compound that is widely used as a flavorant and odorant. The compound is structurally related to benzaldehyde. This compound is popular commercially because of its pleasant woody fragrance. It is derivative of vanillin, from which it is prepared by methylation.Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, , Kurt Bauer, Dorothea Garbe and Horst Surburg "Flavors and Fragrances" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2003. Uses Veratraldehyde can be used as an intermediate in the synthesis of some pharmaceutical drugs including amiquinsin, hoquizil, piquizil, prazosin, quinazoline, tiapamil Tiapamil (INN; also known as dimeditiapramine) is a calcium antagonist or calcium channel blocker. It is an experimental drug that has never been marketed. Tiapamil has been described as an antianginal agent. It exhibits properties o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antihypertensive
Antihypertensives are a class of drugs that are used to treat hypertension (high blood pressure). Antihypertensive therapy seeks to prevent the complications of high blood pressure, such as stroke and myocardial infarction. Evidence suggests that reduction of the blood pressure by 5 mmHg can decrease the risk of stroke by 34% and of ischaemic heart disease by 21%, and can reduce the likelihood of dementia, heart failure, and mortality from cardiovascular disease. There are many classes of antihypertensives, which lower blood pressure by different means. Among the most important and most widely used medications are thiazide diuretics, calcium channel blockers, ACE inhibitors, angiotensin II receptor antagonists (ARBs), and beta blockers. Which type of medication to use initially for hypertension has been the subject of several large studies and resulting national guidelines. The fundamental goal of treatment should be the prevention of the important endpoints of hypert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyldopa
Methyldopa, sold under the brand name Aldomet among others, is a medication used for high blood pressure. It is one of the preferred treatments for high blood pressure in pregnancy. For other types of high blood pressure including hypertensive emergency, very high blood pressure resulting in symptoms other medications are typically preferred. It can be given Oral administration, by mouth or intravenous, injection into a vein. Onset of effects is around 5 hours and they last about a day. Common side effects include sleepiness. More severe side effects include hemolysis, red blood cell breakdown, liver problems, and allergic reactions. Methyldopa is in the alpha-2 adrenergic receptor agonist family of medication. It works by stimulating the brain to decrease the activity of the sympathetic nervous system. Methyldopa was discovered in 1960. It is on the WHO Model List of Essential Medicines, World Health Organization's List of Essential Medicines. Medical uses Methyldopa is us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section Forms below), hence it is stored as an aqueous solution (formalin), which is also used to store animal specimens. It is the simplest of the aldehydes (). The common name of this substance comes from its similarity and relation to formic acid. Formaldehyde is an important precursor to many other materials and chemical compounds. In 1996, the installed capacity for the production of formaldehyde was estimated at 8.7 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Forms Formaldehyde is more complicated than many simple carbon compounds in that it adopts several diverse forms. These compounds can often be used interchangeably and can be interconverted. *Molecular formald ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |