|
Neon-26
Neon (10Ne) possesses three stable isotopes: , , and . In addition, 17 radioactive isotopes have been discovered, ranging from to , all short-lived. The longest-lived is with a half-life of . All others are under a minute, most under a second. The least stable is with a half-life of (). See isotopes of carbon for notes about the measurement. Light radioactive neon isotopes usually decay to fluorine or oxygen, while heavier ones decay to sodium. List of isotopes , - , , style="text-align:right" , 10 , style="text-align:right" , 5 , , [] , proton emission, 2p , , (3/2−) , , , - , , style="text-align:right" , 10 , style="text-align:right" , 6 , , > [ , - , , style="text-align:right" , 10 , style="text-align:right" , 11 , , colspan=3 align=center, Stable , 3/2+ , , ref name="Isotopic Composition of Elements" /> , - , , style="text-align:right" , 10 , style="text-align:right" , 12 , , colspan=3 align=center, Stable , 0+ , , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neon Chart
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, , neuter singular form of (), meaning 'new'. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates. During cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, , neuter singular form of (), meaning 'new'. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates. During cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties. The term isotope is formed from the Greek roots isos ( ἴσος "equal") and topos ( τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd in 1913 in a suggestion to the British chemist Frederick Soddy. The number of protons within the atom's nucleus is called its atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic numbe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Carbon
Carbon (6C) has 15 known isotopes, from to , of which and are stable nuclide, stable. The longest-lived radionuclide, radioisotope is , with a half-life of years. This is also the only carbon radioisotope found in nature—trace quantities are formed cosmogenic nuclide, cosmogenically by the reaction + → + . The most stable artificial radioisotope is , which has a half-life of . All other radioisotopes have half-lives under 20 seconds, most less than 200 milliseconds. The least stable isotope is , with a half-life of . List of isotopes , - , , style="text-align:right" , 6 , style="text-align:right" , 2 , , [] , proton emission, 2p , Subsequently decays by double proton emission to for a net reaction of → + 4 , 0+ , , , - , rowspan=3, , rowspan=3 style="text-align:right" , 6 , rowspan=3 style="text-align:right" , 3 , rowspan=3, , rowspan=3, , β+ () , , rowspan=3, 3/2− , rowspan=3, , rowspan=3, , - , β+α () , Immediately decays ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
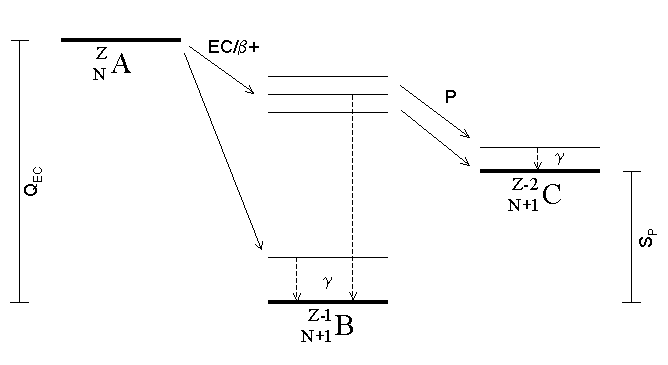
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halo Nucleus
In nuclear physics, an atomic nucleus is called a halo nucleus or is said to have a nuclear halo when it has a core nucleus surrounded by a "halo" of orbiting protons or neutrons, which makes the radius of the nucleus appreciably larger than that predicted by the liquid drop model. Halo nuclei form at the extreme edges of the table of nuclides — the neutron drip line and proton drip line — and have short half-lives, measured in milliseconds. These nuclei are studied shortly after their formation in an ion beam. Typically, an atomic nucleus is a tightly bound group of protons and neutrons. However, in some nuclides, there is an overabundance of one species of nucleon. In some of these cases, a nuclear core and a halo will form. Often, this property may be detected in scattering experiments, which show the nucleus to be much larger than the otherwise expected value. Normally, the cross-section (corresponding to the classical radius) of the nucleus is proportional to the cube roo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Emission
Proton emission (also known as proton radioactivity) is a rare type of radioactive decay in which a proton is ejected from a nucleus. Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay. For a proton to escape a nucleus, the proton separation energy must be negative—the proton is therefore unbound, and tunnels out of the nucleus in a finite time. Proton emission is not seen in naturally occurring isotopes; proton emitters can be produced via nuclear reactions, usually using linear particle accelerators. Although prompt (i.e. not beta-delayed) proton emission was observed from an isomer in cobalt-53 as early as 1969, no other proton-emitting states were found until 1981, when the proton radioactive ground states of lutetium-15 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photoneutron emission and beta-delayed neutron emission. As only a neutron is lost by this process the number of protons remains unchanged, and an atom does not become an atom of a different element, but a different isotope of the same element. Neutrons are also produced in the spontaneous and induced fission of certain heavy nuclides. Spontaneous neutron emission As a consequence of the Pauli exclusion principle, nuclei with an excess of protons or neutrons have a higher average energy per nucleon. Nuclei with a sufficient excess of neutrons have a greater energy than the combination of a free neutron and a nucleus with one less neutron, and therefore can decay by neutron emission. Nuclei which can decay by this process are described as lying beyond the neutron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Neon
Neon (10Ne) possesses three stable isotopes: , , and . In addition, 17 radioactive isotopes have been discovered, ranging from to , all short-lived. The longest-lived is with a half-life of . All others are under a minute, most under a second. The least stable is with a half-life of (). See isotopes of carbon for notes about the measurement. Light radioactive neon isotopes usually decay to fluorine or oxygen, while heavier ones decay to sodium. List of isotopes , - , , style="text-align:right" , 10 , style="text-align:right" , 5 , , [] , proton emission, 2p , , (3/2−) , , , - , , style="text-align:right" , 10 , style="text-align:right" , 6 , , > [ , - , , style="text-align:right" , 10 , style="text-align:right" , 11 , , colspan=3 align=center, Stable , 3/2+ , , ref name="Isotopic Composition of Elements" /> , - , , style="text-align:right" , 10 , style="text-align:right" , 12 , , colspan=3 align=center, Stable , 0+ , , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |