|
Nanocrystalline Material
A nanocrystalline (NC) material is a polycrystalline material with a crystallite size of only a few nanometers. These materials fill the gap between amorphous materials without any long range order and conventional coarse-grained materials. Definitions vary, but nanocrystalline material is commonly defined as a crystallite (grain) size below 100 nm. Grain sizes from 100–500 nm are typically considered "ultrafine" grains. The grain size of a NC sample can be estimated using x-ray diffraction. In materials with very small grain sizes, the diffraction peaks will be broadened. This broadening can be related to a crystallite size using the Scherrer equation (applicable up to ~50 nm), a Williamson-Hall plot, or more sophisticated methods such as the Warren-Averbach method or computer modeling of the diffraction pattern. The crystallite size can be measured directly using transmission electron microscopy. Synthesis Nanocrystalline materials can be prepared in several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycrystalline
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel longulites. Structure The orientation of crystallites can be random with no preferred direction, called random texture, or directed, possibly due to growth and processing conditions. While the structure of a (single) crystal is highly ordered and its lattice is continuous and unbroken, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in-between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are polycrystalline, made of a large number crystallites held together by thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor Deposition
Vacuum deposition is a group of processes used to deposit layers of material atom-by-atom or molecule-by-molecule on a solid surface. These processes operate at pressures well below atmospheric pressure (i.e., vacuum). The deposited layers can range from a thickness of one atom up to millimeters, forming freestanding structures. Multiple layers of different materials can be used, for example to form optical coatings. The process can be qualified based on the vapor source; physical vapor deposition uses a liquid or solid source and chemical vapor deposition uses a chemical vapor. Description The vacuum environment may serve one or more purposes: * reducing the particle density so that the mean free path for collision is long * reducing the particle density of undesirable atoms and molecules (contaminants) * providing a low pressure plasma environment * providing a means for controlling gas and vapor composition * providing a means for mass flow control into the processing chambe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coble Creep
Coble creep, a form of diffusion creep, is a mechanism for deformation of crystalline solids. Contrasted with other diffusional creep mechanisms, Coble creep is similar to Nabarro–Herring creep in that it is dominant at lower stress levels and higher temperatures than creep mechanisms utilizing dislocation glide. Coble creep occurs through the diffusion of atoms in a material along grain boundaries. This mechanism is observed in polycrystals or along the surface in a single crystal, which produces a net flow of material and a sliding of the grain boundaries. Robert L. Coble first reported his theory of how materials creep across grain boundaries and at high temperatures in alumina. Here he famously noticed a different creep mechanism that was more dependent on the size of the grain. The strain rate in a material experiencing Coble creep is given by : \frac \equiv \dot_C = A_C\frac\fracD_0e^ = A_C\frac\fracD_, where : A_c is a geometric prefactor : \sigma is the applied stress ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superplasticity
In materials science, superplasticity is a state in which solid Crystallinity, crystalline material is deformed well beyond its usual breaking point, usually over about 600% during tensile deformation. Such a state is usually achieved at high homologous temperature. Examples of superplastic materials are some fine-grained metals and ceramics. Other non-crystalline materials (amorphous) such as silica glass ("molten glass") and polymers also deform similarly, but are not called superplastic, because they are not crystalline; rather, their deformation is often described as Newtonian fluid. Superplastically deformed material gets thinner in a very uniform manner, rather than forming a "neck" (a local narrowing) that leads to fracture. Also, the formation of microvoids, which is another cause of early fracture, is inhibited. In metals and ceramics, requirements for it being superplastic include a fine grain size (less than approximately 20 micrometres) and a fine dispersion of therma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microstructurally Stable Nanocrystalline Alloys
Microstructurally stable nanocrystalline alloys are alloys that are designed to resist microstructural coarsening under various thermo-mechanical loading conditions. Many applications of metal materials require that they can maintain their structure and strength despite very high temperatures. Efforts to prevent deformations from long term stress, referred to as creep, consist of manipulating alloys to reduce coarsening and migration of individual grains within the metal. The small size of individual metal grains provides high interfacial surface energy which is what prompts coarsening, the increase in grain size, and eventually metallic softening. Nanocrystalline creep is considered to follow the Coble creep mechanism, the diffusion of atoms along grain boundaries at low stress levels and high temperatures. One method used to reduce coarsening, is by employing an alloy in which one component has good solubility with another. Since grain size decreases with high solute concentrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighter members of the carbon group. Exceptions are mostly limited to organolead compounds. Like the lighter members of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminum
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity towards ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy Of Fusion
In thermodynamics, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy resulting from providing energy, typically heat, to a specific quantity of the substance to change its state from a solid to a liquid, at constant pressure. It is the amount of energy required to convert one mole of solid into liquid For example, when melting 1 kg of ice (at 0 °C under a wide range of pressures), 333.55 kJ of energy is absorbed with no temperature change. The heat of solidification (when a substance changes from liquid to solid) is equal and opposite. This energy includes the contribution required to make room for any associated change in volume by displacing its environment against ambient pressure. The temperature at which the phase transition occurs is the melting point or the freezing point, according to context. By convention, the pressure is assumed to be unless otherwise specified. Overview The 'enthalpy' of fusi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strain-hardening
In materials science, work hardening, also known as strain hardening, is the strengthening of a metal or polymer by plastic deformation. Work hardening may be desirable, undesirable, or inconsequential, depending on the context. This strengthening occurs because of dislocation movements and dislocation generation within the crystal structure of the material. Many non-brittle metals with a reasonably high melting point as well as several polymers can be strengthened in this fashion. Alloys not amenable to heat treatment, including low-carbon steel, are often work-hardened. Some materials cannot be work-hardened at low temperatures, such as indium, however others can be strengthened only via work hardening, such as pure copper and aluminum. Undesirable work hardening An example of undesirable work hardening is during machining when early passes of a cutter inadvertently work-harden the workpiece surface, causing damage to the cutter during the later passes. Certain alloys are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
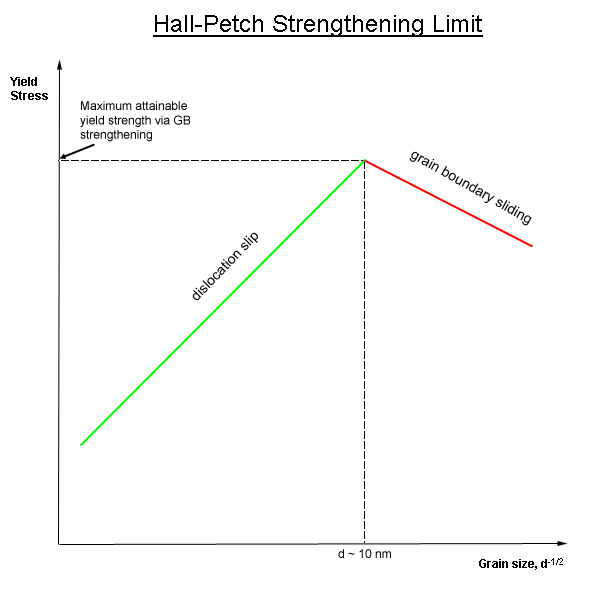
Grain Boundary Strengthening
In materials science, grain-boundary strengthening (or Hall–Petch strengthening) is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable borders for dislocations and that the number of dislocations within a grain has an effect on how stress builds up in the adjacent grain, which will eventually activate dislocation sources and thus enabling deformation in the neighbouring grain as well. So, by changing grain size, one can influence the number of dislocations piled up at the grain boundary and yield strength. For example, heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.W.D. Callister. Fundamentals of Materials Science and Engineering, 2nd ed. Wiley & Sons. pp. 252. Theory In grain-boundary strengthening, the grain boundaries act as pinning points impeding further dislocation propagation. Since the lattice struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rule Of Mixtures
In materials science, a general rule of mixtures is a weighted mean used to predict various properties of a composite material . It provides a theoretical upper- and lower-bound on properties such as the elastic modulus, mass density, ultimate tensile strength, thermal conductivity, and electrical conductivity. In general there are two models, one for axial loading (Voigt model), and one for transverse loading (Reuss model). In general, for some material property E (often the elastic modulus), the rule of mixtures states that the overall property in the direction parallel to the fibers may be as high as : E_c = fE_f + \left(1-f\right)E_m where * f = \frac is the volume fraction of the fibers * E_f is the material property of the fibers * E_m is the material property of the matrix It is a common mistake to believe that this is the upper-bound modulus for Young's modulus. The real upper-bound Young's modulus is larger than E_c given by this formula. Even if both constituents are is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroplating
Electroplating, also known as electrochemical deposition or electrodeposition, is a process for producing a metal coating on a solid substrate through the reduction of cations of that metal by means of a direct electric current. The part to be coated acts as the cathode (negative electrode) of an electrolytic cell; the electrolyte is a solution of a salt of the metal to be coated; and the anode (positive electrode) is usually either a block of that metal, or of some inert conductive material. The current is provided by an external power supply. Electroplating is widely used in industry and decorative arts to improve the surface qualities of objects—such as resistance to abrasion and corrosion, lubricity, reflectivity, electrical conductivity, or appearance. It is used to build up thickness on undersized or worn-out parts, or to manufacture metal plates with complex shape, a process called electroforming. It is used to deposit copper and other conductors in forming printe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |