|
Nosyl
In organosulfur chemistry, a sulfonyl group can refer either to a functional group found primarily in sulfones, or to a substituent obtained from a sulfonic acid by the removal of the hydroxyl group, similarly to acyl groups. Sulfonyl groups can be written as having the general formula , where there are two double bonds between the sulfur and oxygen. Sulfonyl groups can be reduced to the sulfide with DIBALH. Lithium aluminium hydride () reduces some but not all sulfones to sulfides. In inorganic chemistry, when the group is not connected to any carbon atoms, it is referred to as sulfuryl. Examples of sulfonyl group substituents The names of sulfonyl groups typically end in -syl, such as: : See also * Sulfonyl halide * Sulfonamide * Sulfonate In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on sulfur. This group is usually derived from the compound tosyl chloride, (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid, (abbreviated TsOH). The para orientation illustrated (''p''-toluenesulfonyl) is most common, and by convention ''tosyl'' without a prefix refers to the ''p''-toluenesulfonyl group. The toluenesulfonate (or tosylate) group refers to the – (TsO–) group, with an additional oxygen attached to sulfur and open valence on an oxygen. In a chemical name, the term ''tosylate'' may either refer to the salts containing the anion of ''p''-toluenesulfonic acid, (M = alkali metal, , , etc), or it may refer to esters of ''p''-toluenesulfonic acid, TsOR (R = organyl group). Applications For SN2 react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Skeletal Formula
The skeletal formula, or line-angle formula or shorthand formula, of an organic compound is a type of molecular structural formula that serves as a shorthand representation of a molecule's bonding and some details of its molecular geometry. A skeletal formula shows the skeletal structure or skeleton of a molecule, which is composed of the skeletal atoms that make up the molecule. It is represented in two dimensions, as on a piece of paper. It employs certain conventions to represent carbon and hydrogen atoms, which are the most common in organic chemistry. An early form of this representation was first developed by organic chemist August Kekulé, while the modern form is closely related to and influenced by the Lewis structure of molecules and their valence electrons. Hence they are sometimes termed Kekulé structures or Lewis–Kekulé structures. Skeletal formulae have become ubiquitous in organic chemistry, partly because they are relatively quick and simple to draw, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonyl Halide
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl () functional group is singly bonded to a halogen atom. They have the general formula , where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series. Structure Sulfonyl halides have tetrahedral sulfur centres attached to two oxygen atoms, an organic radical, and a halide. In a representative example, methanesulfonyl chloride, the S=O, S−C, and S−Cl bond distances are respectively 142.4, 176.3, and 204.6 pm. Sulfonyl chlorides Sulfonic acid chlorides, or sulfonyl chlorides, are a sulfonyl halide with the general formula . Production Arylsulfonyl chlorides are made industrially in a two-step, one-pot reaction from an arene (in this case, benzene) and chlorosulfuric acid: :C6H6 + HOSO2Cl -> C6H5SO3H + HCl :C6H5SO3H + HOSO2Cl -> C6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide (organic)
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH3. The modern sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Groups
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. Functi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylsulfonylmethane
Methylsulfonylmethane (MSM) is an organosulfur compound with the formula (CH3)2SO2. It is also known by several other names including methyl sulfone and dimethyl sulfone (DMSO2). This colorless solid features the sulfonyl functional group and is the simplest of the sulfones. It is considered relatively inert chemically and is able to resist decomposition at elevated temperatures. It occurs naturally in some primitive plants, is present in small amounts in many foods and beverages, and is marketed as a dietary supplement. It is sometimes used as a cutting agent for illicitly manufactured methamphetamine. It is also commonly found in the atmosphere above marine areas, where it is used as a carbon source by the airborne bacteria ''Afipia''. Oxidation of dimethyl sulfoxide produces the sulfone, both under laboratory conditions and metabolically. Use as a solvent Because of its polarity and thermal stability, MSM has been used industrially as a high-temperature solvent. For example, di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonate
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: :RX + M2SO3 -> RSO3M + MX An alternative is the condensation of a sulfonyl halide with an alcohol in pyridine: :ROH + R' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
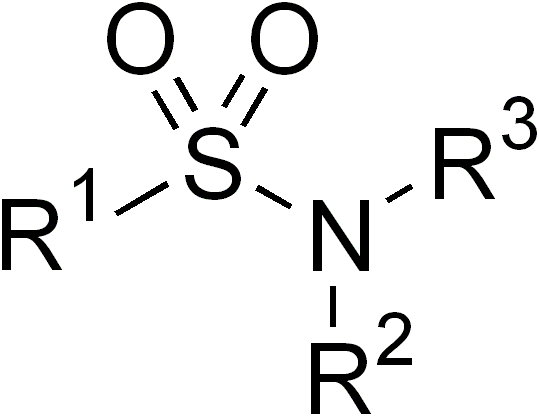
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dansyl Chloride
Dansyl chloride or 5-(DimethylAmino)Naphthalene-1-SulfonYL chloride is a reagent that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green–fluorescent sulfonamide adducts. It can also be made to react with secondary amines. Dansyl chloride is widely used to modify amino acids; specifically, protein sequencing and amino acid analysis. Dansyl chloride may also be denoted DNSC. Likewise, a similar derivative, dansyl amide is known as DNSA. In addition, these protein-DNSC conjugates are sensitive to their immediate environment. This, in combination with their ability to accept energy (as in fluorescence resonance energy transfer) from the amino acid tryptophan, allows this labeling technique to be used in investigating protein folding and dynamics. The fluorescence of these sulfonamide adducts can be enhanced by adding alpha-cyclodextrin. Dansyl chloride is unstable in dimethyl sulfoxide, which should never be used to prep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triflyl
In organic chemistry, the triflyl group (systematic name: trifluoromethanesulfonyl group) is a functional group with the formula and structure . The triflyl group is often represented by –Tf. The related triflate group (trifluoromethanesulfonate) has the formula , and is represented by –OTf. See also * Triflyl azide, TfN3 * Trioctylmethylammonium bis(trifluoromethylsulfonyl)imide, * Comins' reagent * Bis(trifluoromethanesulfonyl)aniline * Triflic anhydride Trifluoromethanesulfonic anhydride, also known as triflic anhydride, is the chemical compound with the formula (CF3SO2)2O. It is the acid anhydride derived from triflic acid. This compound is a strong electrophile, useful for introducing the trif ... (CF3SO2)2O is a very strong triflating agent. References {{organic-chemistry-stub Triflyl compounds Functional groups ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |