|
Sulfonyl Halide
In inorganic chemistry, sulfonyl halide groups occur when a sulfonyl () functional group is singly bonded to a halogen atom. They have the general formula , where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series. Structure Sulfonyl halides have tetrahedral sulfur centres attached to two oxygen atoms, an organic radical, and a halide. In a representative example, methanesulfonyl chloride, the S=O, S−C, and S−Cl bond distances are respectively 142.4, 176.3, and 204.6 pm. Sulfonyl chlorides Sulfonic acid chlorides, or sulfonyl chlorides, are a sulfonyl halide with the general formula . Production Arylsulfonyl chlorides are made industrially in a two-step, one-pot reaction from an arene (in this case, benzene) and chlorosulfuric acid: :C6H6 + HOSO2Cl -> C6H5SO3H + HCl :C6H5SO3H + HOSO2Cl -> C6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry
Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture. Key concepts Many inorganic compounds are ionic compounds, consisting of cations and anions joined by ionic bonding. Examples of salts (which are ionic compounds) are magnesium chloride MgCl2, which consists of magnesium cations Mg2+ and chloride anions Cl−; or sodium oxide Na2O, which consists of sodium cations Na+ and oxide anions O2−. In any salt, the proportions of the ions are such that the electric charges cancel out, so that the bulk compound is e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thionyl Chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons. Thionyl chloride is sometimes confused with sulfuryl chloride, , but the properties of these compounds differ significantly. Sulfuryl chloride is a source of chlorine whereas thionyl chloride is a source of chloride ions. Production The major industrial synthesis involves the reaction of sulfur trioxide and sulfur dichloride: This synthesis can be adapted to the laboratory by heating oleum to slowly distill the sulfur trioxide into a cooled flask of sulfur dichloride. :SO3 + SCl2 -> SOCl2 + SO2 Other methods includ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of two carbon atoms, usually in two separate hydrocarbon substituents. Synthesis and reactions By oxidation of thioethers and sulfoxides Sulfones are typically prepared by organic oxidation of thioethers, often referred to as sulfides. Sulfoxides are intermediates in this route. For example, dimethyl sulfide oxidizes to dimethyl sulfoxide and then to dimethyl sulfone. From SO2 : Sulfur dioxide is a convenient and widely used source of the sulfonyl functional group. Specifically, Sulfur dioxide participates in cycloaddition reactions with dienes. The industrially useful solvent sulfolane is prepared by addition of sulfur dioxide to buta-1,3-diene followed by hydrogenation of the resulting sulfolene. From sulfonyl and sulfuryl halides Sulfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friedel–Crafts Reaction
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution. Alkylation With alkyl halides Friedel–Crafts alkylation involves the alkylation of an aromatic ring. Traditionally, the alkylating agents are alkyl halides. Many alkylating agents can be used instead of alkyl halides. For example, enones and epoxides can be used in presence of protons. Traditionally also, the reaction employs a strong Lewis acid, such as aluminium chloride as catalyst. This reaction suffers from the disadvantage that the product is more nucleophilic than the reactant because alkyl groups are activators for the Friedel–Crafts reaction. Consequently, overalkylation can occur. Steric hindrance can be exploited to limit the number of alkylations, as in the ''t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonate
In organosulfur chemistry, a sulfonate is a salt or ester of a sulfonic acid. It contains the functional group , where R is an organic group. Sulfonates are the conjugate bases of sulfonic acids. Sulfonates are generally stable in water, non-oxidizing, and colorless. Many useful compounds and even some biochemicals feature sulfonates. Sulfonate salts Anions with the general formula are called sulfonates. They are the conjugate bases of sulfonic acids with formula . As sulfonic acids tend to be strong acids, the corresponding sulfonates are weak bases. Due to the stability of sulfonate anions, the cations of sulfonate salts such as scandium triflate have application as Lewis acids. A classic preparation of sulfonates is the Strecker sulfite alkylation, in which an alkali sulfite salt displaces a halide, typically in the presence of an iodine catalyst: :RX + M2SO3 -> RSO3M + MX An alternative is the condensation of a sulfonyl halide with an alcohol in pyridine: :ROH + R' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
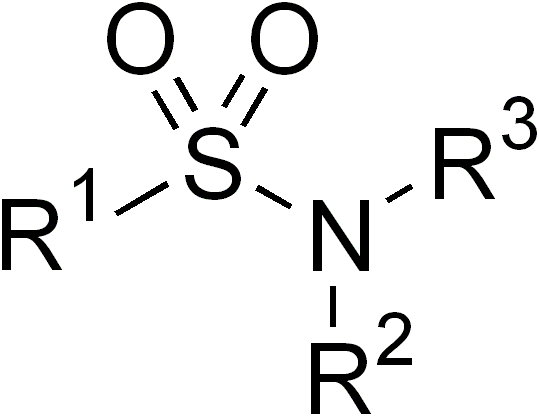
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hinsberg Reaction
The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary amines. The reaction was first described by Oscar Hinsberg in 1890. In this test, the amine is shaken well with Hinsberg reagent in the presence of aqueous alkali (either KOH or NaOH). A reagent containing an aqueous sodium hydroxide solution and benzenesulfonyl chloride is added to a substrate. A primary amine will form a soluble sulfonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulfonamide. A tertiary amine will not react with the original reagent(benzene sulfonyl chloride) and will remain insoluble. After adding dilute acid this insoluble amine is converted to a soluble ammonium salt The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl () functional group bound to a saturated carbon atom. The term ''alcohol'' originally referred to the primary alcohol ethanol (ethyl alcohol), which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula . Simple monoalcohols that are the subject of this article include primary (), secondary () and tertiary () alcohols. The suffix ''-ol'' appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority. When a higher priority group is present in the compound, the prefix ''hydroxy-'' is used in its IUPAC name. The suffix ''-ol'' in non-IUPAC names (such as paracetamol or cholesterol) also typically indicates that the substance is an alcohol. However, some compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reed Reaction
The Reed reaction is a chemical reaction that utilizes light to oxidize hydrocarbons to alkylsulfonyl chlorides. This reaction is employed in modifying polyethylene to give chlorosulfonated polyethylene (CSPE), which noted for its toughness. Commercial implementations Polyethylene is treated with a mixture of chlorine and sulfur dioxide under UV-radiation. Vinylsulfonic acid can also be prepared beginning with the sulfochlorination of chloroethane. Dehydrohalogenation of the product gives vinylsulfonyl chloride, which subsequently is hydrolyzed to give vinylsulfonic acid: :: ::= :: Mechanism The reaction occurs via a free radical mechanism. UV-light initiates homolysis of chlorine, producing a pair of chlorine atoms: Chain initiation: ::::Cl2 -> \nu2Cl. Thereafter a chlorine atom attacks the hydrocarbon chain, freeing hydrogen to form hydrogen chloride and an alkyl free radical. The resulting radical then captures SO2. The resulting sulfonyl radical attacks another c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |