|
Nectin
Nectins and Nectin-like molecules (Necl) are families of cellular adhesion molecules involved in Ca2+-independent cellular adhesion. Nectins are ubiquitously expressed and have adhesive roles in a wide range of tissues such as the adherens junction of epithelia or the chemical synapse of the neuronal tissue. Diversity So far four nectins have been identified in humans, namely nectin-1, nectin-2, nectin-3 and nectin-4. These four family members have also been found in most other well studied mammals. Also, five Necls have been identified, these are: Necl-1, Necl-2, Necl-3, Necl-4 and Necl-5. Structure All nectins and all Necls share the same overall structure defined by three extra cellular immunoglobulin domains, a single transmembrane helix and an intracellular domain. For all nectins the intracellular domain can bind a scaffold protein named afadin (the product of the MLLT4 gene). All nectins and Necls can form homo-cis dimers, meaning a dimer of two alike molecules o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nectin-3
Nectin-3, also known as nectin cell adhesion molecule 3, is a protein that in humans is encoded by the ''NECTIN3'' gene. Nectin-3 belongs to the family of immunoglobulin(Ig)-like cellular adhesion molecules involved in Ca2+-independent cellular adhesion in several tissues during the development and was firstly isolated at the turn of 20th and 21st century. Structure and localization Nectin-3 has three splicing variants, nectin-3α, which is the biggest one, nectin-3β and the smallest variant nectin-3γ. Nectin-3α (same as the other splicing variants) is abundately expressed in testis, on slightly level it is also expressed in heart, brain, liver or kidney. It has been also proved that nectin-3α is together with nectin-2 localize at the junctional complex regions in small intestina absorptive epitelia. Nectin-3γ is also detectable in lung, liver and kidney. Nectin-3 is expressed not only on epithelial cells as another nectins, but there was shown that, as the only membe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PVRL3
Nectin-3, also known as nectin cell adhesion molecule 3, is a protein that in humans is encoded by the ''NECTIN3'' gene. Nectin-3 belongs to the family of immunoglobulin(Ig)-like cellular adhesion molecules involved in Ca2+-independent cellular adhesion in several tissues during the development and was firstly isolated at the turn of 20th and 21st century. Structure and localization Nectin-3 has three splicing variants, nectin-3α, which is the biggest one, nectin-3β and the smallest variant nectin-3γ. Nectin-3α (same as the other splicing variants) is abundately expressed in testis, on slightly level it is also expressed in heart, brain, liver or kidney. It has been also proved that nectin-3α is together with nectin-2 localize at the junctional complex regions in small intestina absorptive epitelia. Nectin-3γ is also detectable in lung, liver and kidney. Nectin-3 is expressed not only on epithelial cells as another nectins, but there was shown that, as the only membe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nectin-4
Nectins and Nectin-like molecules (Necl) are families of cellular adhesion molecules involved in Ca2+-independent cellular adhesion. Nectins are ubiquitously expressed and have adhesive roles in a wide range of tissues such as the adherens junction of epithelia or the chemical synapse of the neuronal tissue. Diversity So far four nectins have been identified in humans, namely nectin-1, nectin-2, nectin-3 and nectin-4. These four family members have also been found in most other well studied mammals. Also, five Necls have been identified, these are: Necl-1, Necl-2, Necl-3, Necl-4 and Necl-5. Structure All nectins and all Necls share the same overall structure defined by three extra cellular immunoglobulin domains, a single transmembrane helix and an intracellular domain. For all nectins the intracellular domain can bind a scaffold protein named afadin (the product of the MLLT4 gene). All nectins and Necls can form homo-cis dimers, meaning a dimer of two alike molecules o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nectin-4
Nectins and Nectin-like molecules (Necl) are families of cellular adhesion molecules involved in Ca2+-independent cellular adhesion. Nectins are ubiquitously expressed and have adhesive roles in a wide range of tissues such as the adherens junction of epithelia or the chemical synapse of the neuronal tissue. Diversity So far four nectins have been identified in humans, namely nectin-1, nectin-2, nectin-3 and nectin-4. These four family members have also been found in most other well studied mammals. Also, five Necls have been identified, these are: Necl-1, Necl-2, Necl-3, Necl-4 and Necl-5. Structure All nectins and all Necls share the same overall structure defined by three extra cellular immunoglobulin domains, a single transmembrane helix and an intracellular domain. For all nectins the intracellular domain can bind a scaffold protein named afadin (the product of the MLLT4 gene). All nectins and Necls can form homo-cis dimers, meaning a dimer of two alike molecules o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nectin-1
Poliovirus receptor-related 1 (PVRL1), also known as nectin-1 and CD111 (formerly herpesvirus entry mediator C, HVEC) is a human protein of the immunoglobulin superfamily (IgSF), also considered a member of the nectins. It is a membrane protein with three extracellular immunoglobulin domains, a single transmembrane helix and a cytoplasmic tail. The protein can mediate Calcium in biology, Ca2+-independent cellular adhesion further characterizing it as IgSF cell adhesion molecule (IgSF CAM). Function PVRL1 is an adhesion molecule found in a wide range of tissues where it localizes in various junctions such as the adherens junction of epithelial tissue or the chemical synapse of neurons. The cytoplasmic tail of PVRL1 can bind the protein MLLT4, afadin which is a scaffolding protein that binds actin. In the chemical synapse PVRL1 interacts with PVRL3 (nectin-3) and both proteins can be found in neuronal tissue already in early stages of brain development as well as in aging brains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PVRL1
Poliovirus receptor-related 1 (PVRL1), also known as nectin-1 and CD111 (formerly herpesvirus entry mediator C, HVEC) is a human protein of the immunoglobulin superfamily (IgSF), also considered a member of the nectins. It is a membrane protein with three extracellular immunoglobulin domains, a single transmembrane helix and a cytoplasmic tail. The protein can mediate Ca2+-independent cellular adhesion further characterizing it as IgSF cell adhesion molecule (IgSF CAM). Function PVRL1 is an adhesion molecule found in a wide range of tissues where it localizes in various junctions such as the adherens junction of epithelial tissue or the chemical synapse of neurons. The cytoplasmic tail of PVRL1 can bind the protein afadin which is a scaffolding protein that binds actin. In the chemical synapse PVRL1 interacts with PVRL3 (nectin-3) and both proteins can be found in neuronal tissue already in early stages of brain development as well as in aging brains. The two proteins have b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MLLT4
Afadin is a protein that in humans is encoded by the ''AFDN'' gene. Function Afadin is a Ras (see HRAS; MIM 190020) target that regulates cell–cell adhesions downstream of Ras activation. It is fused with MLL (MIM 159555) in leukemias caused by t(6;11) translocations (Taya et al., 1998). upplied by OMIMref name="entrez"> Interactions Afadin has been shown to interact with: * BCR gene, * EPHB3, * F11 receptor, * HRAS and * LMO2, * PVRL1, * PVRL3, * Profilin 1, * RAP1A, * RAP1GAP, * SORBS1, * SSX2IP, * Tight junction protein 1 Zonula occludens-1 ZO-1, also known as Tight junction protein-1 is a 220-kD peripheral membrane protein that is encoded by the ''TJP1'' gene in humans. It belongs to the family of ''zonula occludens proteins'' (ZO-1, ZO-2, and ZO-3), which are ti ..., and * USP9X. References Further reading * * * * * * * * * * * * * * * * * * * {{PDB Gallery, geneid=4301 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nectin-2
Poliovirus receptor-related 2 (PVRL2), also known as nectin-2 and CD112 (formerly herpesvirus entry mediator B, HVEB), is a human plasma membrane glycoprotein. Function This gene encodes a single-pass type I membrane glycoprotein with two Ig-like C2-type domains and an Ig-like V-type domain. This protein is one of the plasma membrane components of adherens junctions. It also serves as an entry for certain mutant strains of herpes simplex virus and pseudorabies virus, and it is involved in cell to cell spreading of these viruses. Variations in this gene have been associated with differences in the severity of multiple sclerosis. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. See also * Cluster of differentiation The cluster of differentiation (also known as cluster of designation or classification determinant and often abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules providi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PVRL2
Poliovirus receptor-related 2 (PVRL2), also known as nectin-2 and CD112 (formerly herpesvirus entry mediator B, HVEB), is a human plasma membrane glycoprotein. Function This gene encodes a single-pass type I membrane glycoprotein with two Ig-like C2-type domains and an Ig-like V-type domain. This protein is one of the plasma membrane components of adherens junctions. It also serves as an entry for certain mutant strains of herpes simplex virus and pseudorabies virus, and it is involved in cell to cell spreading of these viruses. Variations in this gene have been associated with differences in the severity of multiple sclerosis. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. See also * Cluster of differentiation The cluster of differentiation (also known as cluster of designation or classification determinant and often abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
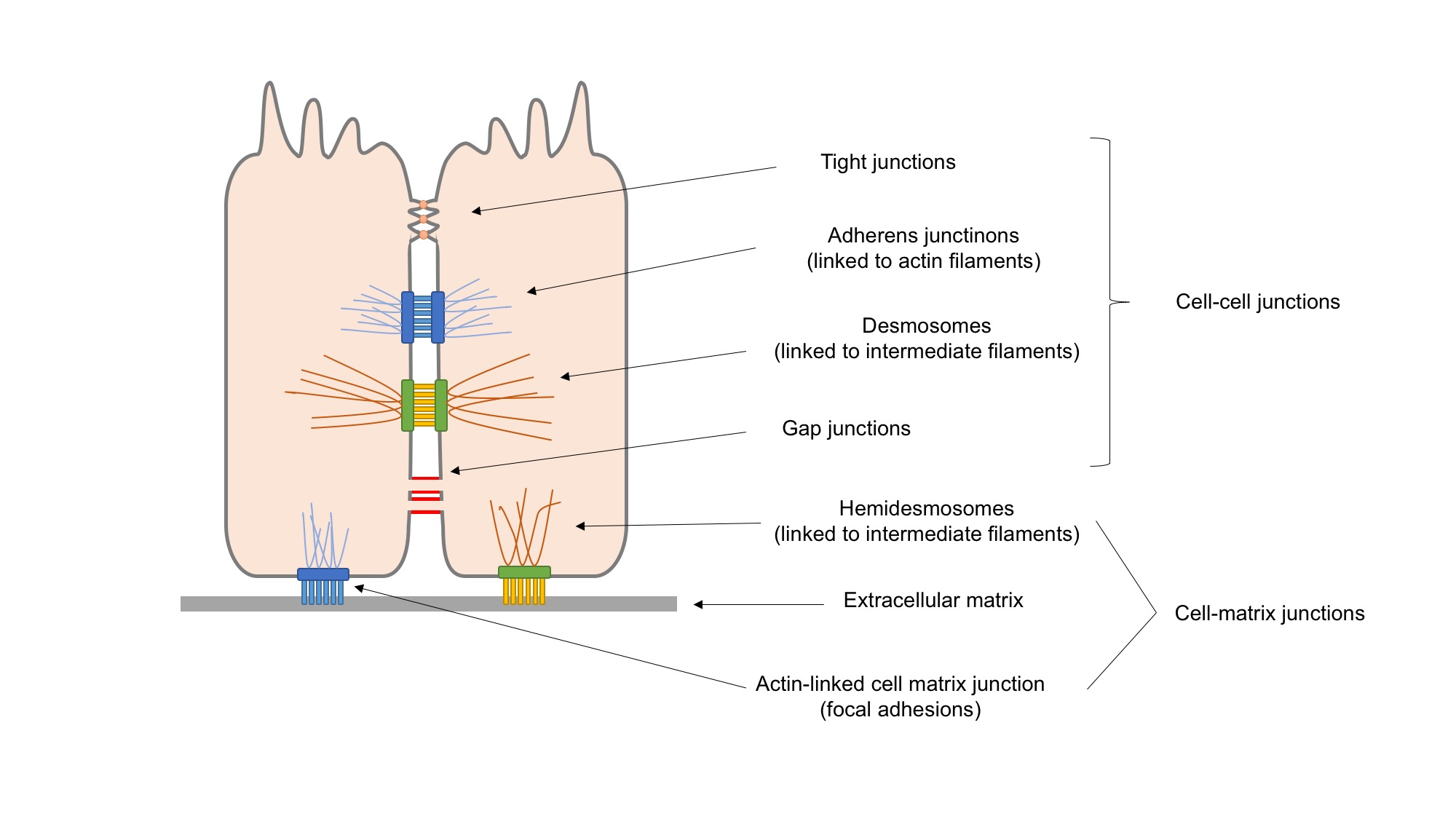
Cellular Adhesion Molecule
Cell adhesion molecules (CAMs) are a subset of cell surface proteins that are involved in the binding of cells with other cells or with the extracellular matrix (ECM), in a process called cell adhesion. In essence, CAMs help cells stick to each other and to their surroundings. CAMs are crucial components in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in generating force and movement and consequently ensuring that organs are able to execute their functions normally. In addition to serving as "molecular glue", CAMs play important roles in the cellular mechanisms of growth, contact inhibition, and apoptosis. Aberrant expression of CAMs may result in a wide range of pathologies, ranging from frostbite to cancer. Structure CAMs are typically single-pass transmembrane receptors and are composed of three conserved domains: an intracellular domain that interacts with the cytoskeleton, a transmembrane domain, and an extrac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellular Adhesion
Cell adhesion is the process by which cells interact and attach to neighbouring cells through specialised molecules of the cell surface. This process can occur either through direct contact between cell surfaces such as cell junctions or indirect interaction, where cells attach to surrounding extracellular matrix, a gel-like structure containing molecules released by cells into spaces between them. Cells adhesion occurs from the interactions between cell-adhesion molecules (CAMs), transmembrane proteins located on the cell surface. Cell adhesion links cells in different ways and can be involved in signal transduction for cells to detect and respond to changes in the surroundings. Other cellular processes regulated by cell adhesion include cell migration and tissue development in multicellular organisms. Alterations in cell adhesion can disrupt important cellular processes and lead to a variety of diseases, including cancer and arthritis. Cell adhesion is also essential for infec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
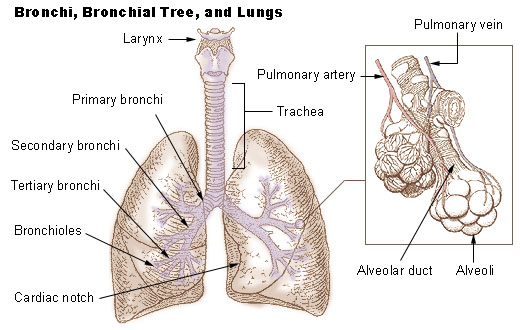
Lung Cancer 1
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side of the heart. Their function in the respiratory system is to extract oxygen from the air and transfer it into the bloodstream, and to release carbon dioxide from the bloodstream into the atmosphere, in a process of gas exchange. Respiration is driven by different muscular systems in different species. Mammals, reptiles and birds use their different muscles to support and foster breathing. In earlier tetrapods, air was driven into the lungs by the pharyngeal muscles via buccal pumping, a mechanism still seen in amphibians. In humans, the main muscle of respiration that drives breathing is the diaphragm. The lungs also provide airflow that makes vocal sounds including human speech possible. Humans have two lungs, one on the left and one on the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |