|
Molecular Encapsulation
In supramolecular chemistry, molecular encapsulation is the confinement of a guest molecule inside the cavity of a supramolecular host molecule (molecular capsule, molecular container or cage compounds). Examples of supramolecular host molecule include carcerands and endohedral fullerenes. Reactivity of guests An important implication of encapsulation is that the guest behaves differently from the way it would when in solution. The guest molecule tends to be unreactive and often has distinctive spectroscopic signatures. Compounds normally highly unstable in solution, such as arynes or cycloheptatetraene, have been isolated at room temperature when molecularly encapsulated. Examples One of the first examples of encapsulating a structure at the molecular level was demonstrated by Donald Cram and coworkers; they were able to isolate highly unstable, antiaromatic cylobutadiene at room temperature by encapsulating it within a hemicarcerand. Isolation of cyclobutadiene allowed chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrobenzene Bound Within Hemicarcerand From Chemical Communications (1997)
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents. Production Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid. This mixture is sometimes called "mixed acid." The production of nitrobenzene is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (Δ''H'' = −117 kJ/mol). World capacity for nitrobenzene in 1985 was about 1,700,000 tonnes. The nitration process involves formation of the nitronium ion (NO2+), followed by an electrophilic aromatic substitution reaction of it with benzene. The nitronium ion is generated by the reaction of nitric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Counting
Electron counting is a formalism used for classifying compounds and for explaining or predicting electronic structure and bonding. Many rules in chemistry rely on electron-counting: *Octet rule is used with Lewis structures for main group elements, especially the lighter ones such as carbon, nitrogen, and oxygen, * 18-electron rule in inorganic chemistry and organometallic chemistry of transition metals, *Hückel's rule for the π-electrons of aromatic compounds, *Polyhedral skeletal electron pair theory for polyhedral cluster compounds, including transition metals and main group elements and mixtures thereof, such as boranes. Atoms are called " electron-deficient" when they have too few electrons as compared to their respective rules, or "hypervalent" when they have too many electrons. Since these compounds tend to be more reactive than compounds that obey their rule, electron counting is an important tool for identifying the reactivity of molecules. Counting rules Two m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Munich
Munich ( ; german: München ; bar, Minga ) is the capital and most populous city of the States of Germany, German state of Bavaria. With a population of 1,558,395 inhabitants as of 31 July 2020, it is the List of cities in Germany by population, third-largest city in Germany, after Berlin and Hamburg, and thus the largest which does not constitute its own state, as well as the List of cities in the European Union by population within city limits, 11th-largest city in the European Union. The Munich Metropolitan Region, city's metropolitan region is home to 6 million people. Straddling the banks of the River Isar (a tributary of the Danube) north of the Northern Limestone Alps, Bavarian Alps, Munich is the seat of the Bavarian Regierungsbezirk, administrative region of Upper Bavaria, while being the population density, most densely populated municipality in Germany (4,500 people per km2). Munich is the second-largest city in the Bavarian dialects, Bavarian dialect area, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Chemistry
Food chemistry is the study of chemical processes and interactions of all biological and non-biological components of foods. The biological substances include such items as meat, poultry, lettuce, beer, milk as examples. It is similar to biochemistry in its main components such as carbohydrates, lipids, and protein, but it also includes areas such as water, vitamins, minerals, enzymes, food additives, flavors, and colors. This discipline also encompasses how products change under certain food processing techniques and ways either to enhance or to prevent them from happening. An example of enhancing a process would be to encourage fermentation of dairy products with microorganisms that convert lactose to lactic acid; an example of preventing a process would be stopping the browning on the surface of freshly cut apples using lemon juice or other acidulated water. History of food chemistry The scientific approach to food and nutrition arose with attention to agricultural chemistry in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanowire
A nanowire is a nanostructure in the form of a wire with the diameter of the order of a nanometre (10−9 metres). More generally, nanowires can be defined as structures that have a thickness or diameter constrained to tens of nanometers or less and an unconstrained length. At these scales, quantum mechanical effects are important—which coined the term "quantum wires". Many different types of nanowires exist, including superconducting (e.g. Yttrium barium copper oxide, YBCO), metallic (e.g. nickel, Ni, platinum, Pt, gold, Au, Ag), semiconducting (e.g. Silicon nanowire, silicon nanowires (SiNWs), indium phosphide, InP, gallium nitride, GaN) and insulating (e.g. Silicon dioxide, SiO2, Titanium dioxide, TiO2). Molecular nanowires are composed of repeating molecular units either organic (e.g. DNA) or inorganic (e.g. Mo6S9−xIx). Characteristics file:SnSe@SWCNT.jpg, upright=1.2, Crystalline 2×2-atom tin selenide nanowire grown inside a single-wall carbon nanotube (tube diamete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complex (chemistry)
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Self-assembly
In chemistry and materials science, molecular self-assembly is the process by which molecules adopt a defined arrangement without guidance or management from an outside source. There are two types of self-assembly: intramolecular and intermolecular. Commonly, the term ''molecular self-assembly'' refers to the former, while the latter is more commonly called ''folding''. Supramolecular systems Molecular self-assembly is a key concept in supramolecular chemistry. This is because assembly of molecules in such systems is directed through non-covalent interactions (e.g., hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi-stacking interactions, and/or electrostatic) as well as electromagnetic interactions. Common examples include the formation of colloids, biomolecular condensates, micelles, vesicles, liquid crystal phases, and Langmuir monolayers by surfactant molecules. Further examples of supramolecular assemblies demonstrate that a variety of d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
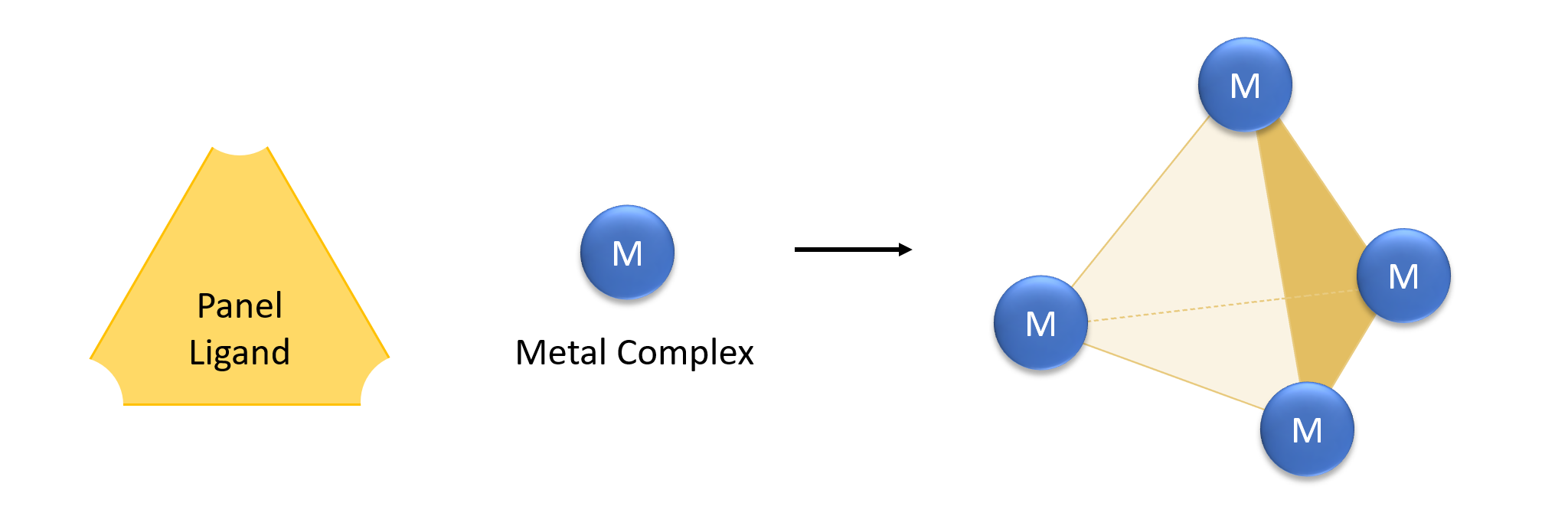
Metallaprism
Coordination cages are three-dimensional ordered structures in solution that act as hosts in host–guest chemistry. They are self-assembled in solution from organometallic precursors, and often rely solely on noncovalent interactions rather than covalent bonds. Coordinate bonds are useful in such supramolecular self-assembly because of their versatile geometries. However, there is controversy over calling coordinate bonds noncovalent, as they are typically strong bonds and have covalent character. The combination of a coordination cage and a guest is a type of inclusion compound. Coordination complexes can be used as "nano-laboratories" for synthesis, and to isolate interesting intermediates. The inclusion complexes of a guest inside a coordination cage show intriguing chemistry as well; often, the properties of the cage will change depending on the guest. Coordination complexes are molecular moieties, so they are distinct from clathrates and metal-organic frameworks. History Chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Counterion
160px, Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion.">cation-exchange_resin.html" ;"title="Polystyrene sulfonate, a cation-exchange resin">Polystyrene sulfonate, a cation-exchange resin, is typically supplied with as the counterion. In chemistry, a counterion (sometimes written as "counter ion", pronounced as such) is the ion that accompanies an Ionic compound, ionic species in order to maintain Electric charge, electric neutrality. In table salt (NaCl, also known as sodium chloride) the sodium ion (positively charged) is the counterion for the chloride ion (negatively charged) and vice versa. A counterion will be more commonly referred to as an anion or a cation, depending on whether it is negatively or positively charged. Thus, the counterion to an anion will be a cation, and vice versa. In biochemistry, counterions are generally vaguely defined. Depending on their charge, proteins are associated with a variety of smaller ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl Complex
A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (''η''5-) bonding mode. The metal–cyclopentadienyl interaction is typically drawn as a single line from the metal center to the center of the Cp ring.Elschenbroich, C. "Organometallics" (2006) Wiley-VCH: Weinheim. Examples ''Bis''cyclopentadienyl complexes are called metallocenes. A famous example of this type of complex is ferrocene (FeCp2), which has many analogues for other metals, such as chromocene (CrCp2), cobaltocene (CoCp2), and nickelocene (NiCp2). When the Cp rings are mutually parallel the compound is known as a sandwich complex. This area of organometallic chemistry was first developed in the 1950s. Bent metallocenes are represented by compounds of the type Cp2Lx Some are catalysts for ethylene polymerization. Metallocenes are often thermally stable, and find use as cata ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)