|
Methoxyl
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula . On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as an electron-donating group, but as an electron-withdrawing group if at the ''meta'' position. At the ''ortho'' position, steric effects are likely to cause a significant alteration in the Hammett equation prediction which otherwise follows the same trend as that of the ''para'' position. Occurrence The simplest of methoxy compounds are methanol and dimethyl ether. Other methoxy ethers include anisole and vanillin. Many alkoxides contain methoxy groups, e.g. tetramethyl orthosilicate and titanium methoxide. Such compounds are often classified as methoxides. Esters with a methoxy group can be referred to as methyl esters, and the —COOCH3 substituent is called a methoxycarbonyl. Biosynthesis In nature, methoxy groups are foun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxy Group
In organic chemistry, a methoxy group is the functional group consisting of a methyl group bound to oxygen. This alkoxy group has the formula . On a benzene ring, the Hammett equation classifies a methoxy substituent at the ''para'' position as an electron-donating group, but as an electron-withdrawing group if at the ''meta'' position. At the ''ortho'' position, steric effects are likely to cause a significant alteration in the Hammett equation prediction which otherwise follows the same trend as that of the ''para'' position. Occurrence The simplest of methoxy compounds are methanol and dimethyl ether. Other methoxy ethers include anisole and vanillin. Many alkoxides contain methoxy groups, e.g. tetramethyl orthosilicate and titanium methoxide. Such compounds are often classified as methoxides. Esters with a methoxy group can be referred to as methyl esters, and the —COOCH3 substituent is called a methoxycarbonyl. Biosynthesis In nature, methoxy groups are found on nucleosi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Methoxide
Titanium ethoxide is a chemical compound with the formula Ti4(OCH2CH3)16. It is a colorless liquid that is soluble in organic solvents but hydrolyzes readily. It is sold commercially as a colorless solution. Alkoxides of titanium(IV) and zirconium(IV) are used in organic synthesis and materials science. They adopt more complex structures than suggested by their empirical formulas. Syntheses Titanium ethoxide is prepared by treating titanium tetrachloride with ethanol in the presence of an amine: :TiCl4 + 4 EtOH + 4 Et3N → Ti(OEt)4 + 4 Et3NHCl The purity of titanium ethoxide is commonly assayed by proton NMR spectroscopy. Ti(OEt)4 1H NMR (90 MHz, chloroform-d, ppm): 4.36 (quartet, 8H, CH2), 1.27 (triplet, 12H, CH3). Structure Both Ti(OEt)4 exist mainly as tetramers with an octahedral coordination environment around the metal centers. There are two types of titanium centers, depending on the number of terminal vs bridging alkoxide ligands. Zr(OEt)4 is structurally similar. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aryl Halide
In organic chemistry, an aryl halide (also known as haloarene) is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications. Preparation The two main preparatory routes to aryl halides are direct halogenation and via diazonium salts. Direct halogenation In the Friedel-Crafts halogenation, Lewis acids serve as catalysts. Many metal chlorides are used, examples include iron(III) chloride or aluminium chloride. The most important aryl halide, chlorobenzene is produced by this route. Monochlorination of benzene is always accompanied by formation of the dichlorobenzene derivatives. Arenes with electron donating groups react with halogens even in the absence of Le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylation
In the chemical sciences, methylation denotes the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and the biological sciences. In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. In vitro methylation of tissue samples is also one method for reducing certain histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals, regulate gene expression, RNA processing and protein function. It has been recognized as a key process underlying epigenetics. Methanogenesis Methanogenesis, the process th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caffeoyl-CoA O-methyltransferase
In enzymology, a caffeoyl-CoA O-methyltransferase () is an enzyme that catalyzes the chemical reaction :S-adenosyl-L-methionine + caffeoyl-CoA \rightleftharpoons S-adenosyl-L-homocysteine + feruloyl-CoA Thus, the two substrates of this enzyme are S-adenosyl methionine and caffeoyl-CoA, whereas its two products are S-adenosylhomocysteine and feruloyl-CoA. A large number of natural products are generated via a step involving this enzyme.Wout Boerjan, John Ralph, Marie Baucher "Lignin Biosynthesis" Annu. Rev. Plant Biol. 2003, vol. 54, pp. 519–46. This enzyme is classified to the family of transferases, specifically those transferring one-carbon group methyltransferases. The systematic name of this enzyme class is S-adenosyl-L-methionine:caffeoyl-CoA 3-O-methyltransferase. Other names in common use include caffeoyl coenzyme A methyltransferase, caffeoyl-CoA 3-O-methyltransferase, and trans-caffeoyl-CoA 3-O-methyltransferase. This enzyme participates in phenylpropanoid biosynthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lignin
Lignin is a class of complex organic polymers that form key structural materials in the support tissues of most plants. Lignins are particularly important in the formation of cell walls, especially in wood and bark, because they lend rigidity and do not rot easily. Chemically, lignins are polymers made by cross-linking phenolic precursors. History Lignin was first mentioned in 1813 by the Swiss botanist A. P. de Candolle, who described it as a fibrous, tasteless material, insoluble in water and alcohol but soluble in weak alkaline solutions, and which can be precipitated from solution using acid. He named the substance “lignine”, which is derived from the Latin word '' lignum'', meaning wood. It is one of the most abundant organic polymers on Earth, exceeded only by cellulose. Lignin constitutes 30% of non-fossil organic carbon on Earth, and 20 to 35% of the dry mass of wood. Lignin is present in red algae, which suggest that the common ancestor of plants and red algae ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechol-O-methyl Transferase
Catechol-''O''-methyltransferase (COMT; ) is one of several enzymes that degrade catecholamines (neurotransmitters such as dopamine, epinephrine, and norepinephrine), catecholestrogens, and various drugs and substances having a catechol structure. In humans, catechol-''O''-methyltransferase protein is encoded by the COMT gene. Two isoforms of COMT are produced: the soluble short form (S-COMT) and the membrane bound long form (MB-COMT). As the regulation of catecholamines is impaired in a number of medical conditions, several pharmaceutical drugs target COMT to alter its activity and therefore the availability of catecholamines. COMT was first discovered by the biochemist Julius Axelrod in 1957. Function Catechol-''O''-methyltransferase is involved in the inactivation of the catecholamine neurotransmitters (dopamine, epinephrine, and norepinephrine). The enzyme introduces a methyl group to the catecholamine, which is donated by S-adenosyl methionine (SAM). Any compound having a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water at phenol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-methyltransferase
An O-methyltransferase (OMT) is a type of methyltransferase enzyme transferring a methyl group on a molecule. Examples are : * Acetylserotonin O-methyltransferase * Apigenin 4'-O-methyltransferase * Caffeate O-methyltransferase * Caffeoyl-CoA O-methyltransferase * Catechol O-methyltransferase * Chlorophenol O-methyltransferase * Columbamine O-methyltransferase * Demethylmacrocin O-methyltransferase * 3'-demethylstaurosporine O-methyltransferase * Demethylsterigmatocystin 6-O-methyltransferase * 3-demethylubiquinone-9 3-O-methyltransferase * 3,7-dimethylquercetin 4'-O-methyltransferase * Fatty-acid O-methyltransferase * Glucuronoxylan 4-O-methyltransferase * 10-hydroxydihydrosanguinarine 10-O-methyltransferase * 12-hydroxydihydrochelirubine 12-O-methyltransferase * 6-hydroxymellein O-methyltransferase * 3'-hydroxy-N-methyl-(S)-coclaurine 4'-O-methyltransferase * 8-hydroxyquercetin 8-O-methyltransferase * Iodophenol O-methyltransferase * Isobutyraldoxime O-methyltransferase * (iso) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
O-methylated Flavonoid
The O-methylated flavonoids or methoxyflavonoids are flavonoids with methylations on hydroxyl groups (methoxy bonds). O-methylation has an effect on the solubility of flavonoids. Enzymes O-methylated flavonoids formation implies the presence of specific O-methyltransferase (OMT) enzymes which accept a variety of substrates. Those enzymes mediate the O-methylation on a specific hydroxyl group, like on 4' (example in ''Catharanthus roseus'') or 3' (example in rice) positions. Those positions can be ortho, meta, para and there can be a special 3-O-methyltransferase for the 3-OH position. Calamondin orange ('' Citrus mitis'') exhibits all of those activities. Plant enzymes * Apigenin 4'-O-methyltransferase * 8-hydroxyquercetin 8-O-methyltransferase * Isoflavone 4'-O-methyltransferase * Isoflavone 7-O-methyltransferase * Isoliquiritigenin 2'-O-methyltransferase * Isoorientin 3'-O-methyltransferase * Kaempferol 4'-O-methyltransferase * Luteolin O-methyltransferase * Methylquerce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
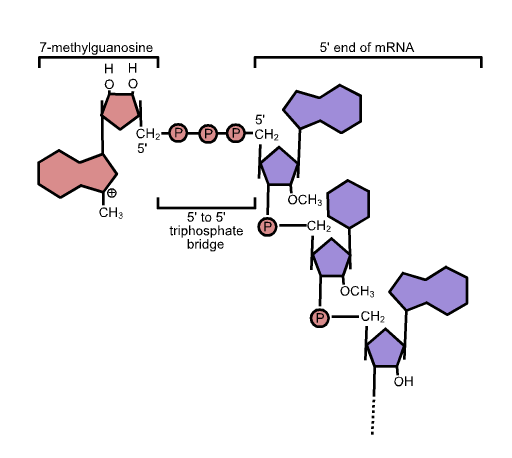
Five-prime Cap
In molecular biology, the five-prime cap (5′ cap) is a specially altered nucleotide on the 5′ end of some primary transcripts such as precursor messenger RNA. This process, known as mRNA capping, is highly regulated and vital in the creation of stable and mature messenger RNA able to undergo translation during protein synthesis. Mitochondrial mRNA and chloroplastic mRNA are not capped. Structure In eukaryotes, the 5′ cap (cap-0), found on the 5′ end of an mRNA molecule, consists of a guanine nucleotide connected to mRNA via an unusual 5′ to 5′ triphosphate linkage. This guanosine is methylated on the 7 position directly after capping ''in vivo'' by a methyltransferase. It is referred to as a 7-methylguanylate cap, abbreviated m7G. In multicellular eukaryotes and some viruses, further modifications exist, including the methylation of the 2′ hydroxy-groups of the first 2 ribose sugars of the 5′ end of the mRNA. cap-1 has a methylated 2′-hydroxy gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2'-O-methylation
2'-''O''-methylation is a common nucleoside modification of RNA, where a methyl group is added to the 2' hydroxyl of the ribose moiety of a nucleoside, producing a methoxy group. 2'-''O''-methylated nucleosides are mostly found in ribosomal RNA and small nuclear RNA and occur in the functionally essential regions of the ribosome and spliceosome. Currently, about 1210 2'-''O''-methylations (2'-''O''-Me) have been identified in mammals and yeast and deposited in RMBase (RNA Modification Base) RMBase (RNA Modification Base) is designed for decoding the landscape of RNA modifications identified from high-throughput sequencing data (MeRIP-seq, m6A-seq, miCLIP, m6A-CLIP, Pseudo-seq, Ψ-seq, CeU-seq, Aza-IP, RiboMeth-seq). It contains ~12 ... database. Having chemical properties intermediate between RNA and DNA, 2'-''O''-methylation is presumed to have been one of the reactive group of RNA molecules on early earth that would have given rise to DNA. A novel method, Nm-REP-seq, was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |