|
Methaneselenol
Methaneselenol is the organoselenium compound with the formula CH3SeH. It is the simplest selenol. A colorless gas, it is notorious for its foul odor. It is prepared by reaction of methyl lithium or a methyl Grignard reagent with selenium followed by protonation of the product The compound is a metabolite. According to IR spectroscopy, νSe-H = 2342 cm−1. For the other homologues, νE-H = 1995 (E = Te), 2606 (E = S), and 3710 cm−1 (E = O) for methanetellurol, methanethiol, and methanol Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ....{{cite journal , doi=10.1080/00945717708069709, title=The Synthesis and the Raman and Infrared Spectra of Methanetellurol, year=1977, last1=Hamada, first1=K., last2=Morishita, first2=H., journal=Synthesis and Reactivity in Inorga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
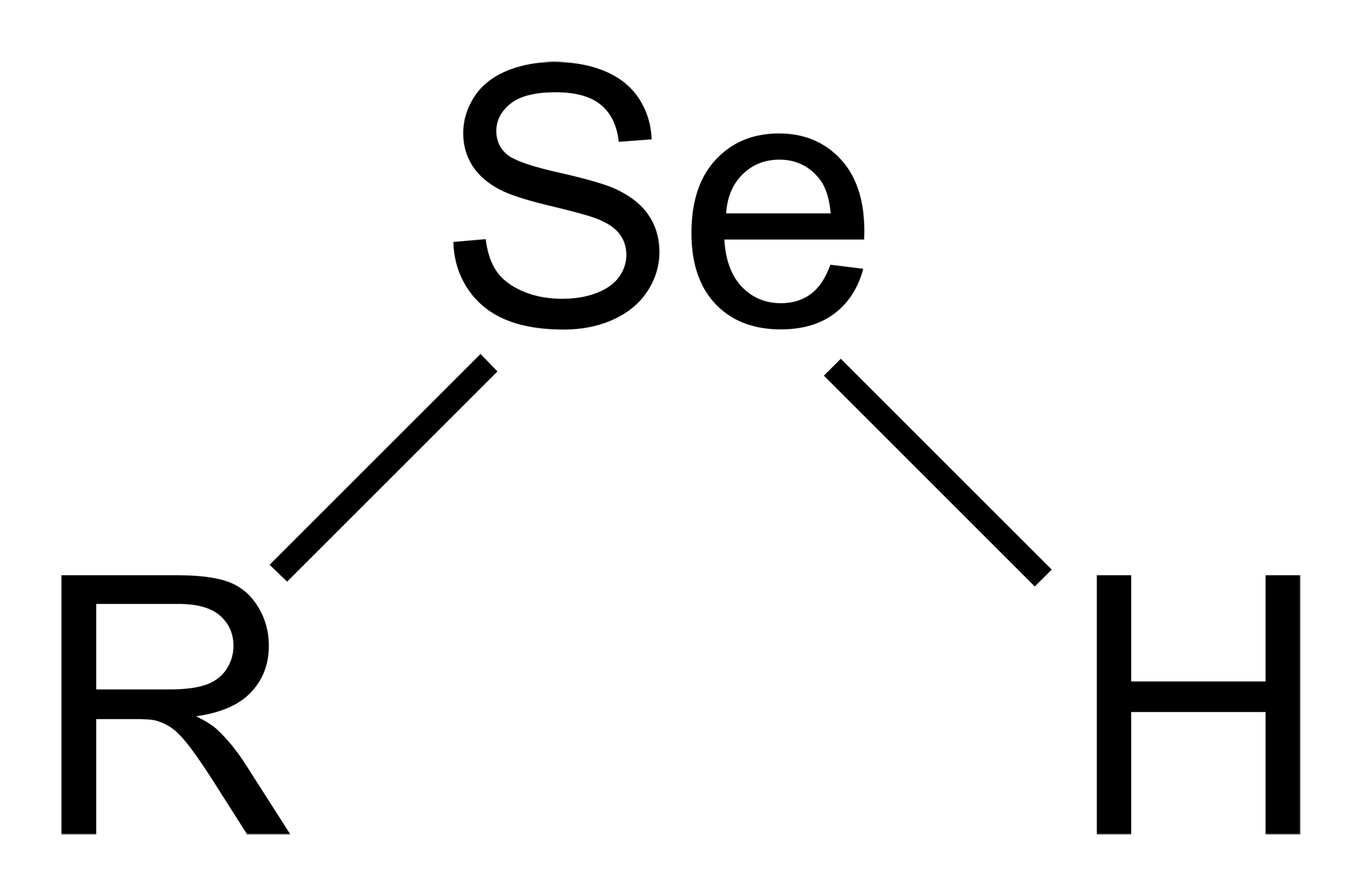
Selenol
Selenols are organic compounds that contain the functional group with the connectivity C–Selenium, Se–H. Selenols are sometimes also called selenomercaptans and selenothiols. Selenols are one of the principal classes of organoselenium compounds. The best known member of the group is the amino acid selenocysteine. Structure, bonding, properties Selenols are structurally similar to thiols, but the C-Se bond is about 8% longer at 196 pm. The C–Se–H angle approaches 90°. The bonding involves almost pure p-orbitals on Se, hence the near 90 angles. The Se–H bond energy is weaker than the S–H bond, consequently selenols are easily oxidized and serve as H-atom donors. The Se-H bond is much weaker than the S-H bond as reflected in their respective bond dissociation energy (BDE). For C6H5Se-H, the BDE is 326 kJ/mol, while for C6H5S-H, the BDE is 368 kJ/mol. Selenol acids are about 1000 times stronger than thiols: the p''K''a of CH3SeH is 5.2 vs 8.3 for CH3SH. Deprotonation affo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoselenium Compound
Organoselenium compounds (or seleno-organic) are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments. Selenium can exist with oxidation state −2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength becomes increasingly weaker (234 kJ/ mol for the C−Se bond and 272 kJ/mol for the C−S bond) and the bond lengths longer (C−Se 198 pm, C−S 181 pm and C−O 141 pm). Selenium compounds are more nucleophilic than the corresponding sulfur compounds and also more acidic. The p''K''a values of XH2 are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxides, the corresponding sel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IR Spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functional groups in solid, liquid, or gaseous forms. It can be used to characterize new materials or identify and verify known and unknown samples. The method or technique of infrared spectroscopy is conducted with an instrument called an infrared spectrometer (or spectrophotometer) which produces an infrared spectrum. An IR spectrum can be visualized in a graph of infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber or wavelength on the horizontal axis. Typical units of wavenumber used in IR spectra are reciprocal centimeters, with the symbol cm−1. Units of IR wavelength are commonly given in micrometers (formerly called "microns"), symbol μm, which are related to the wavenumber in a reciprocal way. A comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanetellurol
Methanetellurol is the organotellurium compound with the formula CH3TeH. It is the simplest organotellurium compound that has been purified in bulk. It is classified as a tellurol. A colorless gas, it decomposes to Te and methane near room temperature. It is prepared by reduction of dimethyl ditelluride using Na/NH3 followed by protonation of the NaTeCH3 with sulfuric acid. Few publications describe this compound as a consequence of its instability and paucity of applications. According to IR spectroscopy, νTe-H = 1995 cm−1. For the lighter homologues, νE-H = 2342 (E = Se), 2606 (E = S), and 3710 cm−1 (E = O) for methaneselenol, methanethiol Methanethiol (also known as methyl mercaptan) is an organosulfur compound with the chemical formula . It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals (including humans ..., and methanol.{{cite journal , doi=10.1080/00945717708069709, title=The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanethiol
Methanethiol (also known as methyl mercaptan) is an organosulfur compound with the chemical formula . It is a colorless gas with a distinctive putrid smell. It is a natural substance found in the blood, brain and feces of animals (including humans), as well as in plant tissues. It also occurs naturally in certain foods, such as some nuts and cheese. It is one of the chemical compounds responsible for bad breath and the smell of flatus. Methanethiol is the simplest thiol and is sometimes abbreviated as MeSH. It is very flammable. Structure and reactions The molecule is tetrahedral at the carbon atom, like methanol. It is a weak acid, with a p''K''a of ~10.4, but is about a hundred thousand times more acidic than methanol. The colorless salt can be obtained in this way: :CH3SH + CH3ONa → CH3SNa + CH3OH The resulting thiolate anion is a strong nucleophile. It can be oxidized to dimethyl disulfide: :2CH3SH + → CH3SSCH3 + H2O Further oxidation takes the disulfide to two mol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals. Occurrence Small amounts of methanol are present in normal, healthy hu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrides
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |