|
Mass Spectra
A mass spectrum is a histogram plot of intensity vs. ''mass-to-charge ratio'' (''m/z'') in a chemical sample, usually acquired using an instrument called a ''mass spectrometer''. Not all mass spectra of a given substance are the same; for example, some mass spectrometers break the analyte molecules into '' fragments''; others observe the intact molecular masses with little fragmentation. A mass spectrum can represent many different types of information based on the type of mass spectrometer and the specific experiment applied. Common fragmentation processes for organic molecules are the ''McLafferty rearrangement'' and ''alpha cleavage''. Straight chain alkanes and alkyl groups produce a typical series of peaks: 29 (CH3CH2+), 43 (CH3CH2CH2+), 57 (CH3CH2CH2CH2+), 71 (CH3CH2CH2CH2CH2+) etc. X-axis: ''m/z'' (mass-to-charge ratio) The x-axis of a mass spectrum represents a relationship between the mass of a given ion and the number of elementary charges that it carries. This is writte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene Ei Ms
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm. History The compound was first isolated in 1837 through a distillation of pine oil by the Polish chemist Filip Walter, who named it ''rétinnaphte''. In 1841, French chemist Henri Étienne Sainte-Claire Deville isolated a hydrocarbon from balsam of Tolu (an aromatic extract from the tropical Colombian tree ''Myroxylon balsamum''), which Deville recognized as similar to W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Whole Number Rule
In chemistry, the whole number rule states that the masses of the isotopes are whole number multiples of the mass of the hydrogen atom. The rule is a modified version of Prout's hypothesis proposed in 1815, to the effect that atomic weights are multiples of the weight of the hydrogen atom. It is also known as the Aston whole number rule after Francis W. Aston who was awarded the Nobel Prize in Chemistry in 1922 "for his discovery, by means of his mass spectrograph, of isotopes, in a large number of non-radioactive elements, and for his enunciation of the whole-number rule." Law of definite proportions The law of definite proportions was formulated by Joseph Proust around 1800 and states that all samples of a chemical compound will have the same elemental composition by mass. The atomic theory of John Dalton expanded this concept and explained matter as consisting of discrete atoms with one kind of atom for each element combined in fixed proportions to form compounds. Prout's hyp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
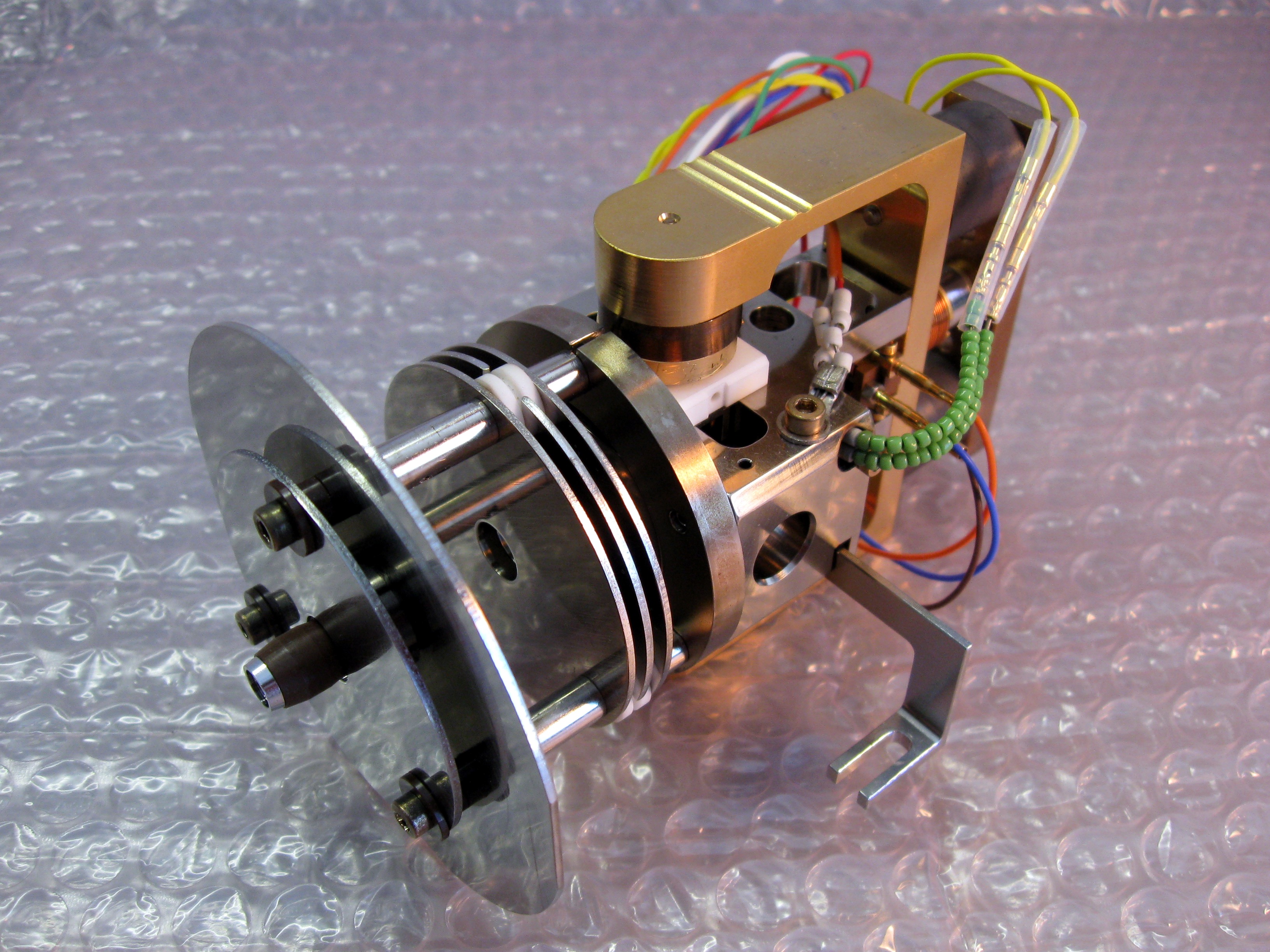
Ion Source
An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines. Electron ionization Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is :M + e^- -> M^ + 2e^- where M is the atom or molecule being ionized, e^- is the electron, and M^ is the resulting ion. The electrons may be created by an arc discharge between a cathode and an anode. An electron beam ion source (EBIS) is used in atomic physics to produce highly charged ions by bombarding atoms with a powerful electron beam. Its principle of operation is shared by the electron beam ion trap. Electron capture ionization Electron capture ionization (ECI) is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form A−•. The reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, etc. In reality, no substance has been found to be 100% pure in its quality, so a substance that is found to be most pure (for some metals, 99% after electrolysis) is called an analyte. See also *Analytical chemistry *Immunoassay *Magnetic immunoassay Magnetic immunoassay (MIA) is a type of diagnostic immunoassay using magnetic beads as labels in lieu of conventional enzymes (ELISA), radioisotopes (RIA) or fluorescent moieties ( fluorescent immunoassays) to detect a specified analyte. MIA involv ... References Analytical chemistry {{Analytical-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accelerator Mass Spectrometry
Accelerator mass spectrometry (AMS) is a form of mass spectrometry that accelerates ions to extraordinarily high kinetic energies before mass analysis. The special strength of AMS among the mass spectrometric methods is its power to separate a rare isotope from an abundant neighboring mass ("abundance sensitivity", e.g. 14C from 12C). The method suppresses molecular isobars completely and in many cases can separate atomic isobars (e.g. 14N from 14C) also. This makes possible the detection of naturally occurring, long-lived radio-isotopes such as 10Be, 36Cl, 26Al and 14C. Their typical isotopic abundance ranges from 10−12 to 10−18. AMS can outperform the competing technique of decay counting for all isotopes where the half-life is long enough. Other advantages of AMS include its short measuring time as well as its ability to detect atoms in extremely small samples. The method Generally, negative ions are created (atoms are ionized) in an ion source. In for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Standard
An internal standard in analytical chemistry is a chemical substance that is added in a constant amount to samples, the blank and calibration standards in a chemical analysis. This substance can then be used for calibration by plotting the ratio of the analyte signal to the internal standard signal as a function of the analyte concentration of the standards. This is done to correct for the loss of analyte during sample preparation or sample inlet. The internal standard is a compound that is very similar, but not identical to the chemical species of interest in the samples, as the effects of sample preparation should, relative to the amount of each species, be the same for the signal from the internal standard as for the signal(s) from the species of interest in the ideal case. Adding known quantities of analyte(s) of interest is a distinct technique called standard addition, which is performed to correct for matrix effects. This ratio for the samples is then used to obtain their anal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Induction Decay
In Fourier transform nuclear magnetic resonance spectroscopy, free induction decay (FID) is the observable NMR signal generated by non-equilibrium nuclear spin magnetization precessing about the magnetic field (conventionally along z). This non-equilibrium magnetization can be created generally by applying a pulse of radio-frequency close to the Larmor frequency of the nuclear spins. If the magnetization vector has a non-zero component in the xy plane, then the precessing magnetisation will induce a corresponding oscillating voltage in a detection coil surrounding the sample. This time-domain signal (a sinusoid) is typically digitised and then Fourier transformed in order to obtain a frequency spectrum of the NMR signal i.e. the NMR spectrum. The duration of the NMR signal is ultimately limited by T2 relaxation, but mutual interference of the different NMR frequencies present also causes the signal to be damped more quickly. When NMR frequencies are well-resolved, as is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fourier Transform Ion Cyclotron Resonance
Fourier-transform ion cyclotron resonance mass spectrometry is a type of mass analyzer (or mass spectrometer) for determining the mass-to-charge ratio (''m''/''z'') of ions based on the cyclotron frequency of the ions in a fixed magnetic field. The ions are trapped in a Penning trap (a magnetic field with electric trapping plates), where they are excited (at their resonant cyclotron frequencies) to a larger cyclotron radius by an oscillating electric field orthogonal to the magnetic field. After the excitation field is removed, the ions are rotating at their cyclotron frequency in phase (as a "packet" of ions). These ions induce a charge (detected as an image current) on a pair of electrodes as the packets of ions pass close to them. The resulting signal is called a free induction decay (FID), transient or interferogram that consists of a superposition of sine waves. The useful signal is extracted from this data by performing a Fourier transform to give a mass spectrum. History FT- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sine Wave
A sine wave, sinusoidal wave, or just sinusoid is a curve, mathematical curve defined in terms of the ''sine'' trigonometric function, of which it is the graph of a function, graph. It is a type of continuous wave and also a Smoothness, smooth periodic function. It occurs often in mathematics, as well as in physics, engineering, signal processing and many other fields. Formulation Its most basic form as a function of time (''t'') is: y(t) = A\sin(2 \pi f t + \varphi) = A\sin(\omega t + \varphi) where: * ''A'', ''amplitude'', the peak deviation of the function from zero. * ''f'', ''frequency, ordinary frequency'', the ''Real number, number'' of oscillations (cycles) that occur each second of time. * ''ω'' = 2''f'', ''angular frequency'', the rate of change of the function argument in units of radians per second. * \varphi, ''phase (waves), phase'', specifies (in radians) where in its cycle the oscillation is at ''t'' = 0. When \varphi is non-zero, the entire waveform appears to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Power (physics)
In physics, power is the amount of energy transferred or converted per unit time. In the International System of Units, the unit of power is the watt, equal to one joule per second. In older works, power is sometimes called ''activity''. Power is a scalar quantity. Power is related to other quantities; for example, the power involved in moving a ground vehicle is the product of the aerodynamic drag plus traction force on the wheels, and the velocity of the vehicle. The output power of a motor is the product of the torque that the motor generates and the angular velocity of its output shaft. Likewise, the power dissipated in an electrical element of a circuit is the product of the current flowing through the element and of the voltage across the element. Definition Power is the rate with respect to time at which work is done; it is the time derivative of work: P =\frac where is power, is work, and is time. If a constant force F is applied throughout a distance x, the wor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frequency Domain
In physics, electronics, control systems engineering, and statistics, the frequency domain refers to the analysis of mathematical functions or signals with respect to frequency, rather than time. Put simply, a time-domain graph shows how a signal changes over time, whereas a frequency-domain graph shows how much of the signal lies within each given frequency band over a range of frequencies. A frequency-domain representation can also include information on the phase shift that must be applied to each sinusoid in order to be able to recombine the frequency components to recover the original time signal. A given function or signal can be converted between the time and frequency domains with a pair of mathematical operators called transforms. An example is the Fourier transform, which converts a time function into a complex valued sum or integral of sine waves of different frequencies, with amplitudes and phases, each of which represents a frequency component. The "spectrum" of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.gif)