|
Methenium
In organic chemistry, methenium (also called methylium, carbenium, methyl cation, or protonated methylene) is a cation with the formula . It can be viewed as a methylene radical (:) with an added proton (), or as a methyl radical (•) with one electron removed. It is a carbocation and an enium ion, making it the simplest of the carbenium ions. Structure Experiments and calculations generally agree that the methenium ion is planar, with threefold symmetry. The carbon atom is a prototypical (and exact) example of sp2 hybridization. Preparation and reactions For mass spectrometry studies at low pressure, methenium can be obtained by ultraviolet photoionization of methyl radical, or by collisions of monatomic cations such as and with neutral methane. In such conditions, it will react with acetonitrile to form the ion . Upon capture of a low-energy electron (less than ), it will spontaneously dissociate. It is seldom encountered as an intermediate in the condensed phase. It is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enium Ion
In chemistry, an onium ion is a cation formally obtained by the protonation of mononuclear parent hydride of a pnictogen (group 15 of the periodic table), chalcogen (group 16), or halogen (group 17). The oldest-known onium ion, and the namesake for the class, is ammonium, , the protonated derivative of ammonia, . The name onium is also used for cations that would result from the substitution of hydrogen atoms in those ions by other groups, such as organic radicals, or halogens; such as tetraphenylphosphonium, . The substituent groups may be divalent or trivalent, yielding ions such as iminium and nitrilium. A simple onium ion has a charge of +1. A larger ion that has two onium ion subgroups is called a double onium ion, and has a charge of +2. A triple onium ion has a charge of +3, and so on. Compounds of an onium cation and some other anion are known as onium compounds or onium salts. Onium ions and onium compounds are inversely analogous to ions and ate complexes: *Lewis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbenium Ion
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with a trivalent carbon that bears a +1 formal charge. In older literature the name carbonium ion was used for this class, but now it refers exclusively to another family of carbocations, the carbonium ions, where the charged carbon is pentavalent. The current definitions were proposed by the chemist George Andrew Olah in 1972, and are now widely accepted. Carbenium ions are generally highly reactive due to having an incomplete octet of electrons; however, certain carbenium ions, such as the tropylium ion, are relatively stable due to the positive charge being delocalised between the carbon atoms. Nomenclature Carbenium ions are classified as primary, secondary, or tertiary depending on whether the number of carbon atoms bonded to the ionized carbon is 1, 2, or 3. (Ions with zero carbons attached to the ionized carbon, such as methenium, , are usually included in the primary class). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocations
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called ' non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanium
In chemistry, ethanium or protonated ethane is a highly reactive positive ion with formula . It can be described as a molecule of ethane () with one extra proton (hydrogen nucleus), that gives it a +1 electric charge. Ethanium is one of the simplest carbonium ions (after methanium ). It was first detected as a rarefied gas in 1960 by S. Wexler and N. Jesse. It easily dissociates into ethenium and molecular hydrogen . Production Ethanium was first detected by infrared spectroscopy among the ions produced by electrical discharges in rarefied methane or ethane gas. Ethanium can also be produced by irradiating methane containing traces of ethane with an electron beam at low pressure (about 2 mmHg). The electron beam first creates methanium and methenium ions. The former rapidly transfer their proton to ethane: : + → + The latter reaction is also observed when , or ions are injected into ethane at somewhat lower pressure. Stability and reactions At about 1 mmHg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanium
In chemistry, methanium is a complex positive ion with formula []+, namely a molecule with one carbon atom covalent bond, bonded to three hydrogen atoms and one hydrogen molecule, bearing a +1 electric charge. It is a superacid and one of the onium ions, indeed the simplest carbonium ion. It is highly unstable and highly reactive even upon having a complete octet, thus granting its superacidic properties. Methanium can be produced in the laboratory as a rarefied gas or as a dilute species in superacids. It was prepared for the first time in 1950 and published in 1952 by Victor Talrose and his assistant Anna Konstantinovna Lyubimova. It occurs as an intermediate species in chemical reactions. The methanium ion is named after methane (), by analogy with the derivation of ammonium ion () from ammonia (). Structure Fluxional methanium can be visualised as a carbenium ion with a molecule of hydrogen interacting with the empty orbital in a 3-center-2-electron bond. The bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Radical
Methyl (also systematically named trihydridocarbon) is an organic compound with the chemical formula (also written as •). It is a metastable colourless gas, which is mainly produced ''in situ'' as a precursor to other hydrocarbons in the petroleum cracking industry. It can act as either a strong oxidant or a strong reductant, and is quite corrosive to metals. Chemical properties Its first ionization potential (yielding the methenium ion, ) is . Redox behaviour The carbon centre in methyl can bond with electron-donating molecules by reacting: : + R• → Because of the capture of the nucleophile (R•), methyl has oxidising character. Methyl is a strong oxidant with organic chemicals. However, it is equally a strong reductant with chemicals such as water. It does not form aqueous solutions, as it reduces water to produce methanol and elemental hydrogen: :2 + 2 → 2 + Structure The molecular geometry of the methyl radical is trigonal planar (bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called ' non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultraviolet
Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm (with a corresponding frequency around 30 PHz) to 400 nm (750 THz), shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules. Short-wave ultraviolet light damages DNA and sterilizes surfaces with which it comes into contac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic groups (indicated by R). Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): :H+ + NH3 -> H4 The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : H4 + B- -> HB + NH3 Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions: :H2O + NH3 OH- + H4 The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkane
In organic chemistry Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ..., an alkane, or paraffin (a historical trivial name that also has other meanings), is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula . The alkanes range in complexity from the simplest case of methane (), where ''n'' = 1 (sometimes called the parent molecule), to arbitrarily large and complex molecules, like Higher alkanes#Nonatetracontane to tetrapentacontane, pentacontane () or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane (). The International Union of Pure and Applied Chemistry (IUPAC) defines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
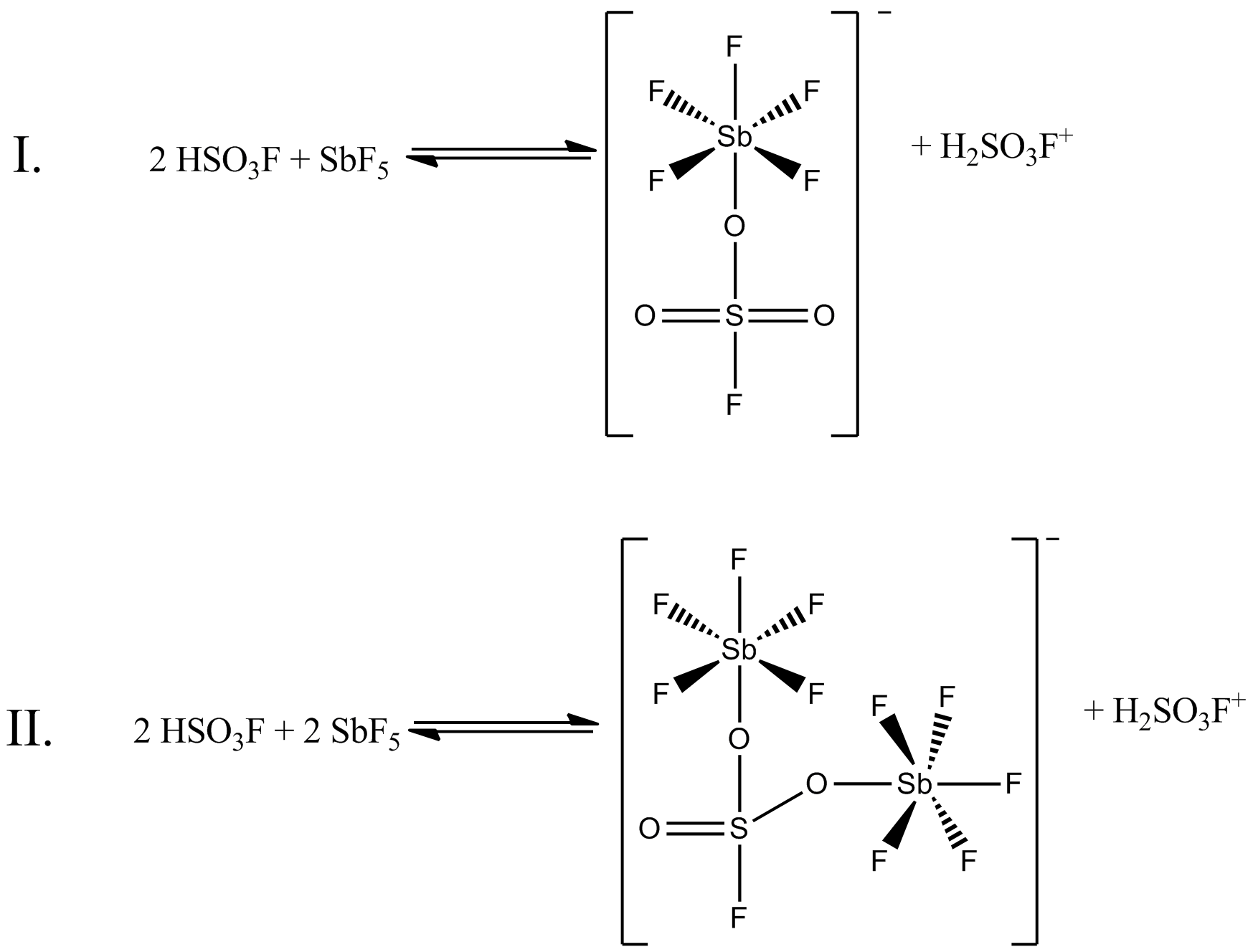
Magic Acid
Magic acid (FSO3H·SbF5) is a superacid consisting of a mixture, most commonly in a 1:1 molar ratio, of fluorosulfuric acid (HSO3F) and antimony pentafluoride (SbF5). This conjugate Brønsted– Lewis superacid system was developed in the 1960s by the George Olah lab at Case Western Reserve University, and has been used to stabilize carbocations and hypercoordinated carbonium ions in liquid media. Magic acid and other superacids are also used to catalyze isomerization of saturated hydrocarbons, and have been shown to protonate even weak bases, including methane, xenon, halogens, and molecular hydrogen. History The term "superacid" was first used in 1927 when James Bryant Conant found that perchloric acid could protonate ketones and aldehydes to form salts in nonaqueous solution. The term itself was coined by R. J. Gillespie later, after Conant combined sulfuric acid with fluorosulfuric acid, and found the solution to be several million times more acidic than sulfuric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not classed as organic). It is produced mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The skeleton is linear with a short distance of 1.16 Å. Acetonitrile was first prepared in 1847 by the French chemist Jean-Baptiste Dumas. Applications Acetonitrile is used mainly as a solvent in the purification of butadiene in refineries. Specifically, acetonitrile is fed into the top of a distillation column filled with hydrocarbons including butadiene, and as the acetonitrile falls down through the column, it absorbs the butadiene which is then sent from the bottom of the tower to a second separating tower. Heat is then employed in the separatin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |