|
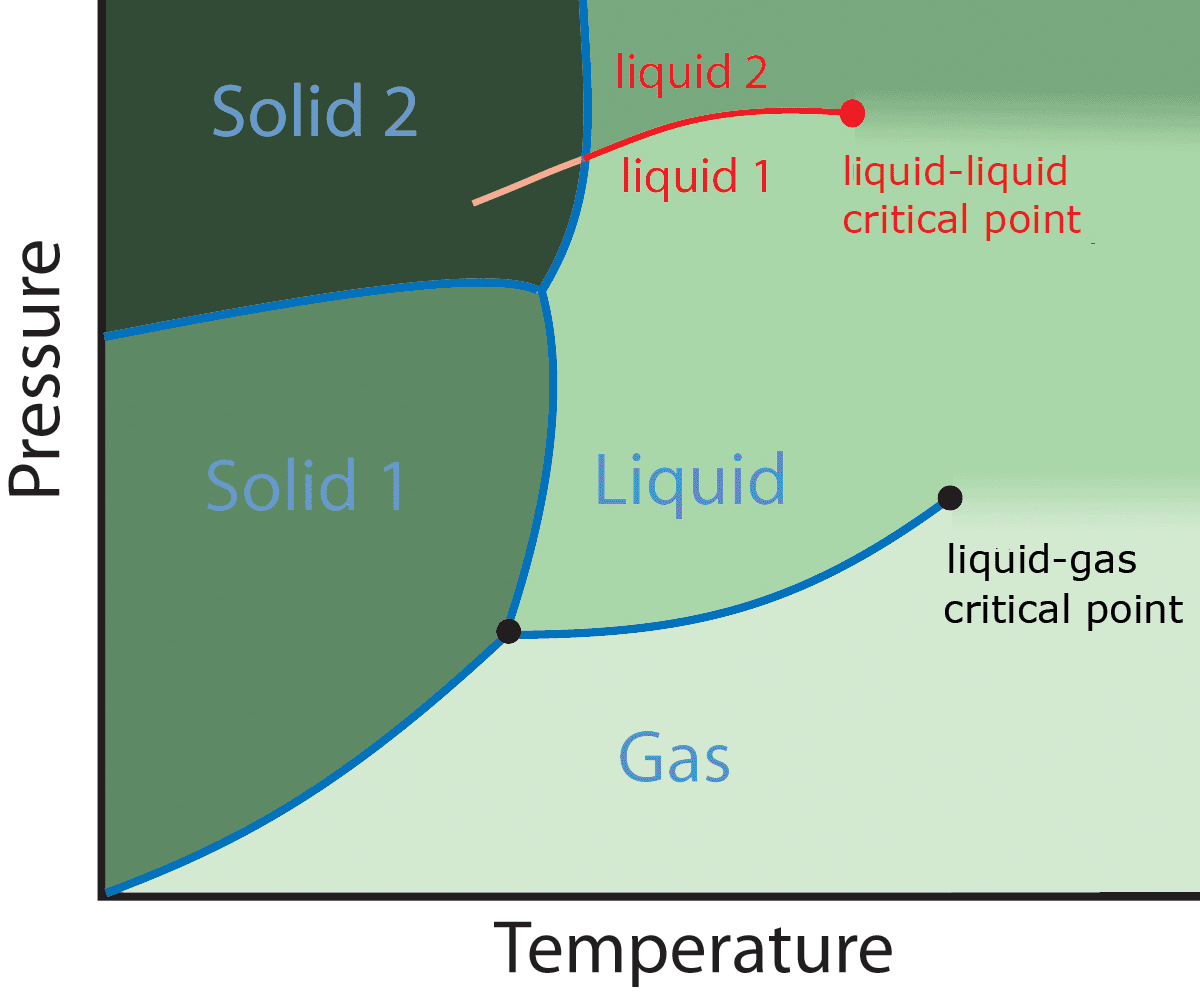
Liquid–liquid Critical Point
A liquid–liquid critical point (or LLCP) is the endpoint of a liquid–liquid phase transition line (LLPT); it is a critical point where two types of local structures coexist at the exact ratio of unity. This hypothesis was first developed by Peter Poole, Francesco Sciortino, Uli Essmann and H. Eugene Stanley in Boston to obtain a quantitative understanding of the huge number of anomalies present in water. Near a liquid–liquid critical point, there is always a competition between two alternative local structures. For instance, in supercooled water, two types of local structures have been predicted: a low-density local configuration (LD) and a high-density local configuration (HD), so above the critical pressure, the liquid is composed by a majority of HD local structure, while below the critical pressure a higher fraction of LD local configurations is present. The ratio between HD and LD configurations is determined according to the thermodynamic equilibrium of the system, wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyamorphism
Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics (e.g. polymers). However, polyamorphism requires ''two distinct'' amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition. Overview Even though amorphous materials exhibit no long-range periodic atomic ordering, there is still significant and varied local structure at inter-atomic length scales (see structure of liquids and glasses). Different local structures can produce amorphous phases of the same chemical composition with different physical properties such as density. In several cases sharp transitions have been observed between two different density amor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Point (thermodynamics)
In thermodynamics, a critical point (or critical state) is the end point of a phase equilibrium curve. The most prominent example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapor can coexist. At higher temperatures, the gas cannot be liquefied by pressure alone. At the critical point, defined by a ''critical temperature'' ''T''c and a ''critical pressure'' ''p''c, phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition ( Curie temperature) in the absence of an external magnetic field. Liquid–vapor critical point Overview For simplicity and clarity, the generic notion of ''critical point'' is best introduced by discussing a specific example, the vapor–liquid critical point. This was the first critical point to be discovered, and it is still the best known and most studied one. The fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Francesco Sciortino
Francesco Sciortino (born December 29, 1960) is an Italian physicist and Full Professor at Sapienza University of Rome. He has made seminal contributions to statistical physics, including the thermodynamic and dynamic theory of complex fluids like water, colloids, colloidal-polymer mixtures, patchy particles, and DNA-based materials. He is one of the original proponents of the "second liquid critical point" theory of water. Education Sciortino was awarded a Ph.D. in Physics from the University of Palermo in 1989 working on the coupling between biomolecules and the solvent, and focusing on the role of water's hydrogen bond cooperativity in supramolecular arrangement of biomolecules. After the Ph.D., Sciortino became Research Assistant in the Center for polymer studies of Boston University, working in the group of Prof. H. Eugene Stanley. Academic career Following his postdoctoral research position in Boston, in 1992 Sciortino became a researcher in Centro di Ricerca, Svilu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boston
Boston (), officially the City of Boston, is the state capital and most populous city of the Commonwealth of Massachusetts, as well as the cultural and financial center of the New England region of the United States. It is the 24th- most populous city in the country. The city boundaries encompass an area of about and a population of 675,647 as of 2020. It is the seat of Suffolk County (although the county government was disbanded on July 1, 1999). The city is the economic and cultural anchor of a substantially larger metropolitan area known as Greater Boston, a metropolitan statistical area (MSA) home to a census-estimated 4.8 million people in 2016 and ranking as the tenth-largest MSA in the country. A broader combined statistical area (CSA), generally corresponding to the commuting area and including Providence, Rhode Island, is home to approximately 8.2 million people, making it the sixth most populous in the United States. Boston is one of the oldest ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral
In geometry, a tetrahedron (plural: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular faces, six straight edges, and four vertex corners. The tetrahedron is the simplest of all the ordinary convex polyhedra and the only one that has fewer than 5 faces. The tetrahedron is the three-dimensional case of the more general concept of a Euclidean simplex, and may thus also be called a 3-simplex. The tetrahedron is one kind of pyramid, which is a polyhedron with a flat polygon base and triangular faces connecting the base to a common point. In the case of a tetrahedron the base is a triangle (any of the four faces can be considered the base), so a tetrahedron is also known as a "triangular pyramid". Like all convex polyhedra, a tetrahedron can be folded from a single sheet of paper. It has two such nets. For any tetrahedron there exists a sphere (called the circumsphere) on which all four vertices lie, and another sph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Phenomena
In physics, critical phenomena is the collective name associated with the physics of critical points. Most of them stem from the divergence of the correlation length, but also the dynamics slows down. Critical phenomena include scaling relations among different quantities, power-law divergences of some quantities (such as the magnetic susceptibility in the ferromagnetic phase transition) described by critical exponents, universality, fractal behaviour, and ergodicity breaking. Critical phenomena take place in second order phase transitions, although not exclusively. The critical behavior is usually different from the mean-field approximation which is valid away from the phase transition, since the latter neglects correlations, which become increasingly important as the system approaches the critical point where the correlation length diverges. Many properties of the critical behavior of a system can be derived in the framework of the renormalization group. In order to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Transitions
In chemistry, thermodynamics, and other related fields, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, and in rare cases, plasma. A phase of a thermodynamic system and the states of matter have uniform physical properties. During a phase transition of a given medium, certain properties of the medium change as a result of the change of external conditions, such as temperature or pressure. This can be a discontinuous change; for example, a liquid may become gas upon heating to its boiling point, resulting in an abrupt change in volume. The identification of the external conditions at which a transformation occurs defines the phase transition point. Types of phase transition At the phase transition point for a substance, for instance the boiling point, the two phases involved - liquid and vapor, have identic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)