|
Liquid Scintillation Counting
Liquid scintillation counting is the measurement of radioactive activity of a sample material which uses the technique of mixing the active material with a liquid scintillator (e.g. zinc sulfide), and counting the resultant photon emissions. The purpose is to allow more efficient counting due to the intimate contact of the activity with the scintillator. It is generally used for alpha particle or beta particle detection. Technique Samples are dissolved or suspended in a "cocktail" containing a solvent (historically aromatic organics such as xylene or toluene, but more recently less hazardous solvents are used), typically some form of a surfactant, and "fluors" or scintillators which produce the light measured by the detector. Scintillators can be divided into primary and secondary phosphors, differing in their luminescence properties. Beta particles emitted from the isotopic sample transfer energy to the solvent molecules: the Aromaticity, π cloud of the aromatic ring absorbs the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
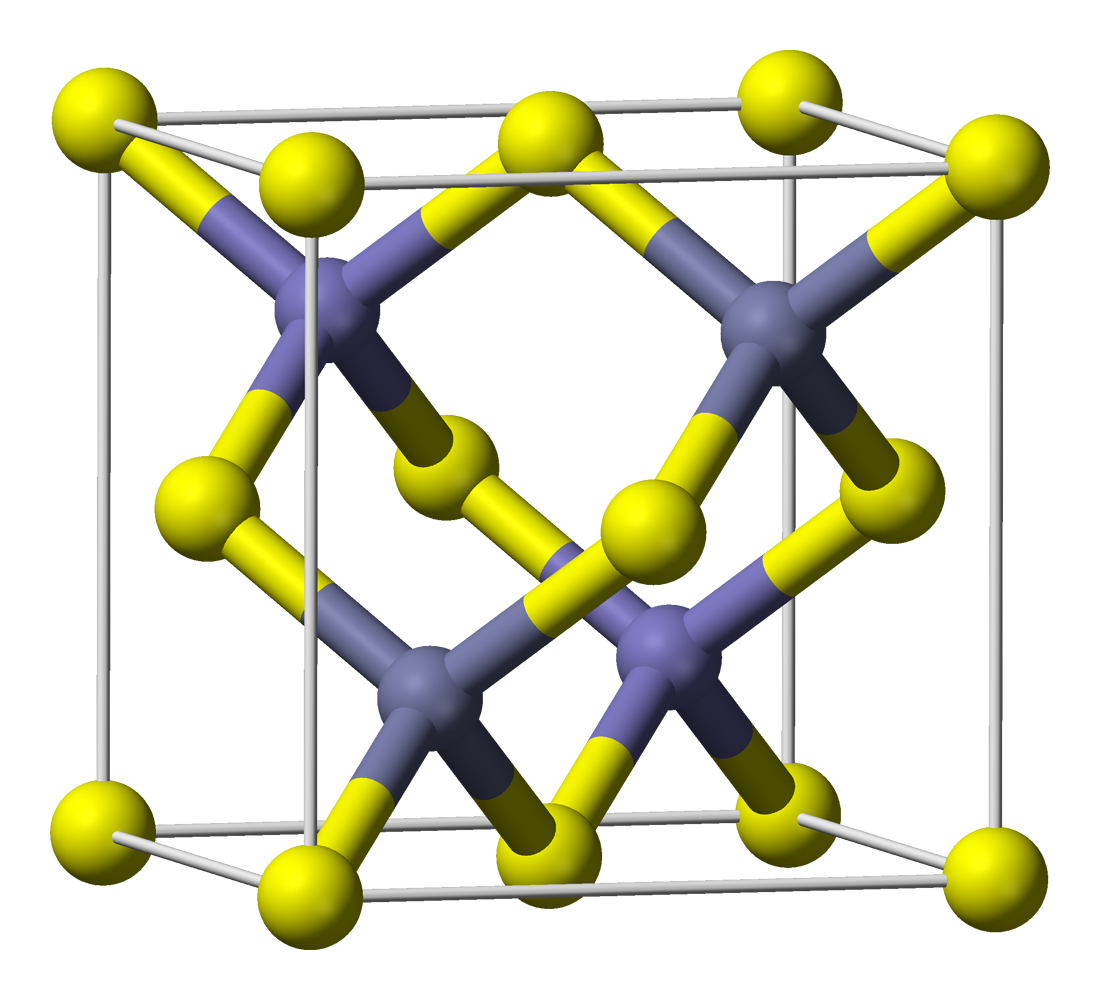
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparency and translucency, transparent, and it is used as a window for visible light, visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism (materials science), polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. Applications Luminescent mate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Translucent
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable light scattering by particles, scattering of light. On a macroscopic scale (one in which the dimensions are much larger than the wavelengths of the photons in question), the photons can be said to follow Snell's law. Translucency (also called translucence or translucidity) is the physical property of allowing light to pass through the material (with or without scattering of light). It allows light to pass through but the light does not necessarily follow Snell's law on the macroscopic scale; the photons may be scattered at either of the two interfaces, or internally, where there is a change in the index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cherenkov Radiation
Cherenkov radiation () is electromagnetic radiation emitted when a charged particle (such as an electron) passes through a dielectric medium (such as distilled water) at a speed greater than the phase velocity (speed of propagation of a wavefront in a medium) of light in that medium. A classic example of Cherenkov radiation is the characteristic blue glow of an underwater nuclear reactor. Its cause is similar to the cause of a sonic boom, the sharp sound heard when faster-than-sound movement occurs. The phenomenon is named after Soviet physicist Pavel Cherenkov. History The radiation is named after the Soviet scientist Pavel Cherenkov, the 1958 Nobel Prize winner, who was the first to detect it experimentally under the supervision of Sergey Vavilov at the Lebedev Institute in 1934. Therefore, it is also known as Vavilov–Cherenkov radiation. Cherenkov saw a faint bluish light around a radioactive preparation in water during experiments. His doctorate thesis was on lumin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aqueous Solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are '' hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chloride. In an aqueous solution the hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Yttrium-90
Yttrium-90 () is a radioactive isotope of yttrium. Yttrium-90 has found a wide range of uses in radiation therapy to treat some forms of cancer Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po .... Along with other isotopes of yttrium, it is sometimes called radioyttrium. Decay undergoes beta particles emissions/decay (beta decay, β− decay) to zirconium-90 with a half-life of 64.1 hours and a decay energy of 2.28 MeV with an average beta energy of 0.9336 MeV. It also produces 0.01% 1.7 MeV photons during its decay process to the 0+ state of 90Zr, followed by pair production. The interaction between emitted electrons and matter can lead to the emission of Bremsstrahlung radiation. Production Yttrium-90 is produced by the nuclear decay of strontium-90 which has a half-l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphorus-32
Phosphorus-32 (32P) is a radioactive isotope of phosphorus. The nucleus of phosphorus-32 contains 15 protons and 17 neutrons, one more neutron than the most common isotope of phosphorus, phosphorus-31. Phosphorus-32 only exists in small quantities on Earth as it has a short half-life of 14 days and so decays rapidly. Phosphorus is found in many organic molecules, and so, phosphorus-32 has many applications in medicine, biochemistry, and molecular biology where it can be used to trace phosphorylated molecules (for example, in elucidating metabolic pathways) and radioactively label DNA and RNA. Decay Phosphorus-32 has a short half-life of 14.268 days and decays into sulfur-32 by beta decay as shown in this nuclear equation: : 1.709 MeV of energy is released from this decay. The kinetic energy of the electron varies with an average of approximately 0.5 MeV and the remainder of the energy is carried by the nearly undetectable electron antineutrino. In comparison to oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidizing agent, oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and . However, the nature of fre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Isotopes Of Phosphorus
Although phosphorus (15P) has 22 isotopes from 26P to 47P, only 31P is stable, thus phosphorus is considered a monoisotopic element. The longest-lived radioactive isotopes are 33P with a half-life of 25.34 days and 32P with a half-life of 14.268 days. All others have half-lives of under 2.5 minutes, most under a second. The least stable known isotope is 47P, with a half-life of 2 milliseconds. List of isotopes , -id=Phosphorus-26 , rowspan=3, 26P , rowspan=3 style="text-align:right" , 15 , rowspan=3 style="text-align:right" , 11 , rowspan=3, 26.01178(21)# , rowspan=3, 43.6(3) ms , β+ (62.9%) , 26Si , rowspan=3, (3)+ , rowspan=3, , - , β+, p (35.1%) , 25Al , - , β+, 2p (1.99%) , 24Mg , -id=Phosphorus-26m , style="text-indent:1em", 26mP , colspan=3 style="text-indent:2em", 164.4(1) keV , 115(8) ns , IT , 26P , (1+) , , -id=Phosphorus-27 , rowspan=2, 27P , rowspan=2 style="text-align:right" , 15 , rowspan=2 style="text-align:right" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tritium
Tritium () or hydrogen-3 (symbol T or H) is a rare and radioactive isotope of hydrogen with a half-life of ~12.33 years. The tritium nucleus (t, sometimes called a ''triton'') contains one proton and two neutrons, whereas the nucleus of the common isotope hydrogen-1 (''protium'') contains one proton and no neutrons, and that of non-radioactive hydrogen-2 ('' deuterium'') contains one proton and one neutron. Tritium is the heaviest particle-bound isotope of hydrogen. It is one of the few nuclides with a distinct name. The use of the name hydrogen-3, though more systematic, is much less common. Naturally occurring tritium is extremely rare on Earth. The atmosphere has only trace amounts, formed by the interaction of its gases with cosmic rays. It can be produced artificially by irradiation of lithium or lithium-bearing ceramic pebbles in a nuclear reactor and is a low-abundance byproduct in normal operations of nuclear reactors. Tritium is used as the energy source in radio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Line Noise
In electronics, noise is an unwanted disturbance in an electrical signal. Noise generated by electronic devices varies greatly as it is produced by several different effects. In particular, noise is inherent in physics and central to thermodynamics. Any conductor with electrical resistance will generate thermal noise inherently. The final elimination of thermal noise in electronics can only be achieved cryogenically, and even then quantum noise would remain inherent. Electronic noise is a common component of noise in signal processing. In communication systems, noise is an error or undesired random disturbance of a useful information signal in a communication channel. The noise is a summation of unwanted or disturbing energy from natural and sometimes man-made sources. Noise is, however, typically distinguished from interference, for example in the signal-to-noise ratio (SNR), signal-to-interference ratio (SIR) and signal-to-noise plus interference ratio (SNIR) measures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Coincidence Circuit
In physics and electrical engineering, a coincidence circuit or coincidence gate is an electronic device with one output and two (or more) inputs. The output activates only when the circuit receives signals within a time window accepted as ''at the same time'' and in parallel at both inputs. Coincidence circuits are widely used in particle detectors and in other areas of science and technology. Walther Bothe shared the Nobel Prize for Physics in 1954 "...for his discovery of the method of coincidence and the discoveries subsequently made by it." Bruno Rossi invented the electronic coincidence circuit for implementing the coincidence method. History Bothe and Geiger, 1924-1925 In his Nobel Prize lecture,{{cite web , url=http://nobelprize.org/nobel_prizes/physics/laureates/1954/bothe-lecture.html , title=Nobel Lecture , author=Bothe, Walther , year=1954 , publisher= Nobel Foundation Bothe described how he had implemented the coincidence method in an experiment on Compton s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Photo Multiplier
A photomultiplier is a device that converts incident photons into an electrical signal. Kinds of photomultiplier include: * Photomultiplier tube, a vacuum tube converting incident photons into an electric signal. Photomultiplier tubes (PMTs for short) are members of the class of vacuum tubes, and more specifically vacuum phototubes, which are extremely sensitive detectors of light in the ultraviolet, visible, and near-infrared ranges of the electromagnetic spectrum. ** Magnetic photomultiplier, developed by the Soviets in the 1930s. ** Electrostatic photomultiplier, a kind of photomultiplier tube demonstrated by Jan Rajchman of RCA Laboratories in Princeton, NJ in the late 1930s which became the standard for all future commercial photomultipliers. The first mass-produced photomultiplier, the Type 931, was of this design and is still commercially produced today. * Silicon photomultiplier, a solid-state device converting incident photons into an electric signal. Silicon photomultipli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |