|
Linear Ion Trap
The linear ion trap (LIT) is a type of ion trap mass spectrometer. In a LIT, ions are confined radially by a two-dimensional radio frequency (RF) field, and axially by stopping potentials applied to end electrodes. LITs have high injection efficiencies and high ion storage capacities. History One of the first LITs was constructed in 1969, by Dierdre A. Church, who bent linear quadrupoles into closed circle and racetrack geometries and demonstrated storage of 3 He+ and H+ ions for several minutes. Earlier, Drees and Paul described a circular quadrupole. However, it was used to produce and confine a plasma, not to store ions. In 1989, Prestage, Dick, and Malecki described that ions could be trapped in the linear quadrupole trap system to enhance ion-molecule reactions, thus it can be used to study spectroscopy of stored ions. How it works The LIT uses a set of quadrupole rods to confine ions radially and a static electrical potential on the end electrodes to confine the ions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage
Voltage, also known as electric pressure, electric tension, or (electric) potential difference, is the difference in electric potential between two points. In a static electric field, it corresponds to the work needed per unit of charge to move a test charge between the two points. In the International System of Units, the derived unit for voltage is named ''volt''. The voltage between points can be caused by the build-up of electric charge (e.g., a capacitor), and from an electromotive force (e.g., electromagnetic induction in generator, inductors, and transformers). On a macroscopic scale, a potential difference can be caused by electrochemical processes (e.g., cells and batteries), the pressure-induced piezoelectric effect, and the thermoelectric effect. A voltmeter can be used to measure the voltage between two points in a system. Often a common reference potential such as the ground of the system is used as one of the points. A voltage can represent either a source ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Duty Cycle
A duty cycle or power cycle is the fraction of one period in which a signal or system is active. Duty cycle is commonly expressed as a percentage or a ratio. A period is the time it takes for a signal to complete an on-and-off cycle. As a formula, a duty cycle (%) may be expressed as: :D = \frac \times 100\% Equally, a duty cycle (ratio) may be expressed as: :D = \frac where D is the duty cycle, PW is the pulse width (pulse active time), and T is the total period of the signal. Thus, a 60% duty cycle means the signal is on 60% of the time but off 40% of the time. The "on time" for a 60% duty cycle could be a fraction of a second, a day, or even a week, depending on the length of the period. Duty cycles can be used to describe the percent time of an active signal in an electrical device such as the power switch in a switching power supply or the firing of action potentials by a living system such as a neuron. The duty factor for periodic signal expresses the same notion, but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrospray Ionization
Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol. It is especially useful in producing ions from macromolecules because it overcomes the propensity of these molecules to fragment when ionized. ESI is different from other ionization processes (e.g. matrix-assisted laser desorption/ionization (MALDI)) since it may produce multiple-charged ions, effectively extending the mass range of the analyser to accommodate the kDa-MDa orders of magnitude observed in proteins and their associated polypeptide fragments. Mass spectrometry using ESI is called electrospray ionization mass spectrometry (ESI-MS) or, less commonly, electrospray mass spectrometry (ES-MS). ESI is a so-called 'soft ionization' technique, since there is very little fragmentation. This can be advantageous in the sense that the molecular ion (or more accurately a pseudo molecular ion) is almost alw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paul Trap
A quadrupole ion trap or paul trap is a type of ion trap that uses dynamic electric fields to trap charged particles. They are also called radio frequency (RF) traps or Paul traps in honor of Wolfgang Paul, who invented the device and shared the Nobel Prize in Physics in 1989 for this work. It is used as a component of a mass spectrometer or a trapped ion quantum computer. Overview A charged particle, such as an atomic or molecular ion, feels a force from an electric field. It is not possible to create a static configuration of electric fields that traps the charged particle in all three directions (this restriction is known as Earnshaw's theorem). It is possible, however, to create an ''average'' confining force in all three directions by use of electric fields that change in time. To do so, the confining and anti-confining directions are switched at a rate faster than it takes the particle to escape the trap. The traps are also called "radio frequency" traps because the switch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FTMS
Fourier-transform ion cyclotron resonance mass spectrometry is a type of mass analyzer (or mass spectrometer) for determining the mass-to-charge ratio (''m''/''z'') of ions based on the ion cyclotron resonance, cyclotron frequency of the ions in a fixed magnetic field. The ions are trapped in a Penning trap (a magnetic field with electric trapping plates), where they are excited (at their resonant cyclotron frequencies) to a larger cyclotron radius by an oscillating electric field orthogonal to the magnetic field. After the excitation field is removed, the ions are rotating at their cyclotron frequency in phase (as a "packet" of ions). These ions induce a charge (detected as an image current) on a pair of electrodes as the packets of ions pass close to them. The resulting signal is called a free induction decay (FID), transient or interferogram that consists of a superposition of sine waves. The useful signal is extracted from this data by performing a Fourier transform to give a ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated accordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analog Signal
An analog signal or analogue signal (see spelling differences) is any continuous signal representing some other quantity, i.e., ''analogous'' to another quantity. For example, in an analog audio signal, the instantaneous signal voltage varies continuously with the pressure of the sound waves. In contrast, a digital signal represents the original time-varying quantity as a sampled sequence of quantized values which imposes some bandwidth and dynamic range constraints on the representation. The term ''analog signal'' usually refers to electrical signals; however, mechanical, pneumatic, hydraulic and other systems may also convey or be considered analog signals. Representation An analog signal uses some property of the medium to convey the signal's information. For example, an aneroid barometer uses rotary position as the signal to convey pressure information. In an electrical signal, the voltage, current, or frequency of the signal may be varied to represent the information. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
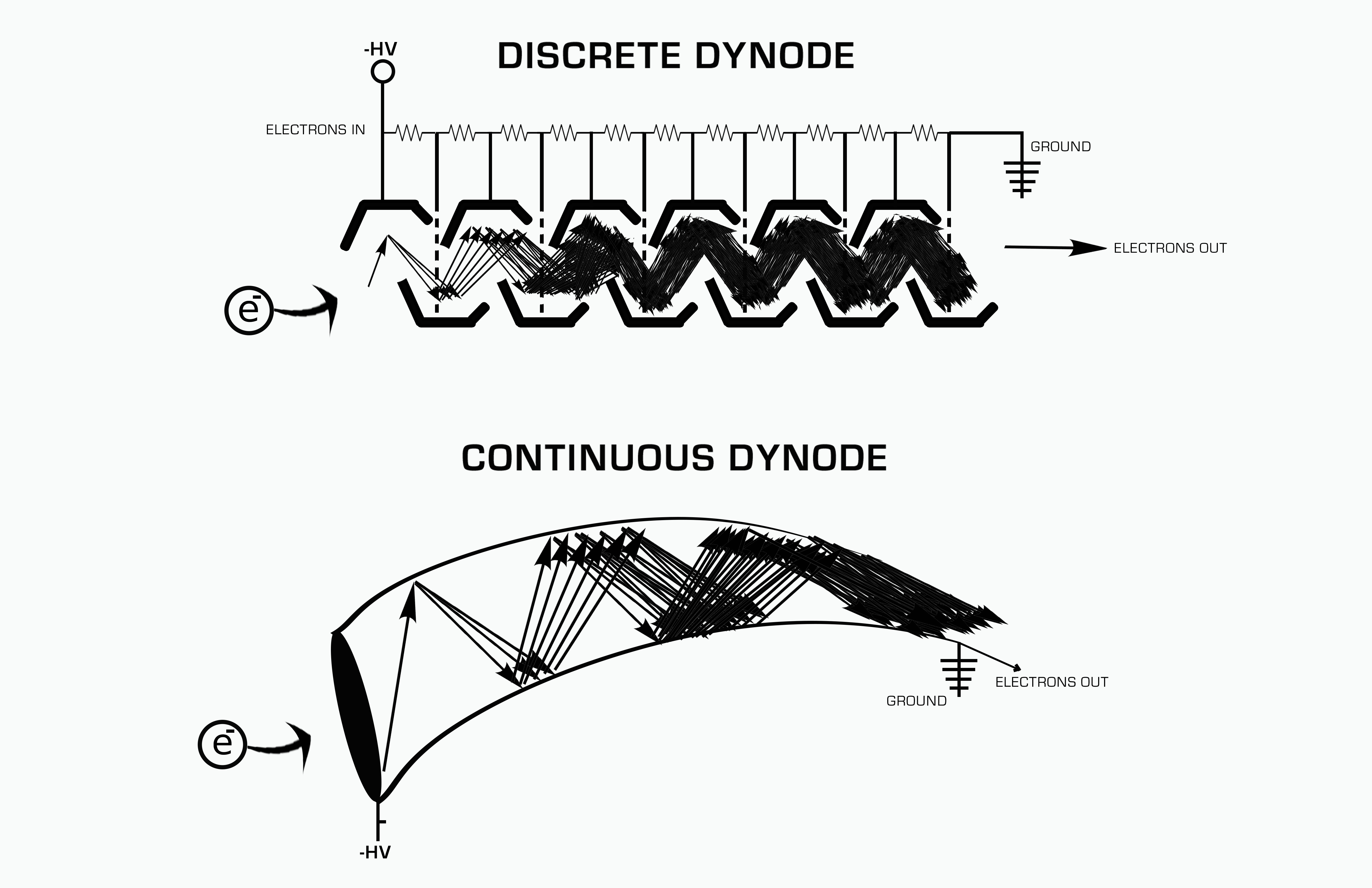
Electron Multiplier
An electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary-emissive material, induce emission of roughly 1 to 3 electrons. If an electric potential is applied between this metal plate and yet another, the emitted electrons will accelerate to the next metal plate and induce secondary emission of still more electrons. This can be repeated a number of times, resulting in a large shower of electrons all collected by a metal anode, all having been triggered by just one. History In 1930, Russian physicist Leonid Aleksandrovitch Kubetsky proposed a device which used photocathodes combined with dynodes, or secondary electron emitters, in a single tube to remove secondary electrons by increasing the electric potential through the device. The electron multiplier can use any number of dynodes in total, which use a coefficient, σ, and created a gain of σn where n is the number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Electrons
Secondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated by other radiation (the ''primary'' radiation). This radiation can be in the form of ions, electrons, or photons with sufficiently high energy, i.e. exceeding the ionization potential. Photoelectrons can be considered an example of secondary electrons where the primary radiation are photons; in some discussions photoelectrons with higher energy (>50 eV) are still considered "primary" while the electrons freed by the photoelectrons are "secondary". Applications Secondary electrons are also the main means of viewing images in the scanning electron microscope (SEM). The range of secondary electrons depends on the energy. Plotting the inelastic mean free path as a function of energy often shows characteristics of the "universal curve" familiar to electron spectroscopists and surface analysts. This distance is on the order of a few nanometers in metals and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynode
A dynode is an electrode in a vacuum tube A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric potential difference has been applied. The type known as ... that serves as an electron multiplier through secondary emission. The first tube to incorporate a dynode was the Dynatron oscillator, dynatron, an ancestor of the magnetron, which used a single dynode.Albert W. Hull, E. F. Hennelly and F. R. Elder, The Dynatron Detector -- a new heterodyne receiver for continuous and modulated wavesProceedings of the Institute of Radio EngineersVol. 10, No. 5 (Oct. 1922), pages 320-343 Photomultiplier and video camera tubes generally include a series of dynodes, each at a more positive electrical potential than its predecessor. Secondary emission occurs at the surface of each dynode. Such an arrangement is able to amplify the tiny current emitted by the ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrum
A mass spectrum is a histogram plot of intensity vs. ''mass-to-charge ratio'' (''m/z'') in a chemical sample, usually acquired using an instrument called a ''mass spectrometer''. Not all mass spectra of a given substance are the same; for example, some mass spectrometers break the analyte molecules into '' fragments''; others observe the intact molecular masses with little fragmentation. A mass spectrum can represent many different types of information based on the type of mass spectrometer and the specific experiment applied. Common fragmentation processes for organic molecules are the ''McLafferty rearrangement'' and ''alpha cleavage''. Straight chain alkanes and alkyl groups produce a typical series of peaks: 29 (CH3CH2+), 43 (CH3CH2CH2+), 57 (CH3CH2CH2CH2+), 71 (CH3CH2CH2CH2CH2+) etc. X-axis: ''m/z'' (mass-to-charge ratio) The x-axis of a mass spectrum represents a relationship between the mass of a given ion and the number of elementary charges that it carries. This is writte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)




.jpg)