|
Lanthanide Chloride
Lanthanide chlorides are a group of chemical compounds that can form between a lanthanide element (from lanthanum to lutetium) and chlorine. The lanthanides in these compounds are usually in the +2 and +3 oxidation states, although compounds with lanthanides in lower oxidation states exist. Lanthanide dichlorides Divalent chlorides are formed by neodymium, samarium, europium, dysprosium, thulium and ytterbium. They can be prepared by reducing the trivalent chloride with lithium metal/naphthalene in tetrahydrofuran: : LnCl3 + Li → LnCl2 + LiCl (Ln=Nd,Sm,Eu) Reducing the chloride with the metal or hydrogen is also possible: : 2 LnCl3 + Ln → 3 LnCl2 (Ln=Nd,Sm,Eu?,Dy,Tm,Yb) : 2 LnCl3 + H2 → 2 LnCl2 + 2 HCl (Ln=Nd,Sm,Eu,Dy,Tm,Yb) Lanthanide trichlorides The lanthanide trichlorides can generally be prepared by dissolving the oxide or carbonate with hydrochloric acid. They are produced commercially by carbothermic reaction of the oxide. To produce the anhydrous forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, the usual oxidation state is +3. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the Ancient Greek (), meaning 'to lie hidden'. Although it is classified as a rare earth element, lanthanum is the 28th most abund ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cerium Trichloride
Cerium(III) chloride (CeCl3), also known as cerous chloride or cerium trichloride, is a compound of cerium and chlorine. It is a white hygroscopic salt; it rapidly absorbs water on exposure to moist air to form a hydrate, which appears to be of variable composition, though the heptahydrate CeCl3·7H2O is known. It is highly soluble in water, and (when anhydrous) it is soluble in ethanol and acetone. Preparation of waterless CeCl3 Simple rapid heating of the hydrate alone may cause small amounts of hydrolysis. A useful form of anhydrous CeCl3 can be prepared if care is taken to heat the heptahydrate gradually to over many hours under vacuum. This may or may not contain a little CeOCl from hydrolysis, but it is suitable for use with organolithium and Grignard reagents. Pure anhydrous CeCl3 can be made by dehydration of the hydrate either by slowly heating to with 4–6 equivalents of ammonium chloride under high vacuum, or by heating with an excess of thionyl chloride ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lutetium Trichloride
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals and also a hydroscopic hexahydrate LuCl3·6H2O. Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications Reactions Pure lutetium metal can be produced from lutetium(III) chloride by heating it together with elemental calcium: :2 LuCl3 + 3 Ca → 2 Lu + 3 CaCl2 See also * Lutetium (177Lu) chloride Lutetium (177Lu) chloride is a radioactive compound used for the radiolabeling of pharmaceutical molecules, aimed either as an anti-cancer therapy or for scintigraphy (medical imaging). Text was copied from this source under the copyright of t ... References Lutetium compounds Chlorides Lanthanide halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ytterbium Trichloride
Ytterbium(III) chloride ( Yb Cl3) is an inorganic chemical compound. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. It is poisonous if injected, and mildly toxic by ingestion. It is an experimental teratogen, known to irritate the skin and eyes. When heated to decomposition it emits toxic fumes of Cl−. History The synthesis of YbCl3 was first reported by Jan Hoogschagen in 1946. It is now a commercially available source of Yb3+ ions and therefore of significant chemical interest. Chemical properties The valence electron configuration of Yb+3 (from YbCl3) is 4''f''135''s''25''p''6, which has crucial implications for the chemical behaviour of Yb+3. Also, the size of Yb+3 governs its catalytic behaviour and biological applications. For example, while both Ce+3 and Yb+3 have a single unpaired ''f'' electron, Ce+3 is much larger than Yb+3 because lanthanides become much smaller with increasing effective nuclear charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thulium Trichloride
Thulium(III) chloride or thulium trichloride is as an inorganic salt composed of thulium and chlorine with the formula In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ... TmCl3. It forms yellow crystals. Thulium(III) chloride has the YCl3 ( AlCl3) layer structure with octahedral thulium ions.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford Science Publications Reactions The hydrated form of thulium(III) chloride can be obtained by adding thulium(III) oxide to concentrated hydrochloric acid. Thulium(III) chloride reacts with strong bases to make thulium(III) oxide. References Thulium compounds Chlorides Lanthanide halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erbium Trichloride
Erbium(III) chloride is a violet solid with the formula . It is used in the preparation of erbium metal. Preparation Anhydrous erbium(III) chloride can be produced by the ammonium chloride route. In the first step, erbium(III) oxide is heated with ammonium chloride to produce the ammonium salt of the pentachloride: : In the second step, the ammonium chloride salt is converted to the trichloride by heating in a vacuum at 350-400 ºC: : Structural data Erbium(III) chloride forms crystals of the type, with monoclinic crystals and the point group ''C''2/m. Erbium(III) chloride hexahydrate also forms monoclinic crystals with the point group of ''P''2/''n'' (''P''2/''c'') - ''C''42h. In this compound, erbium is octa-coordinated to form ions with the isolated completing the structure. Optical properties Erbium(III) chloride solutions show a negative nonlinear absorption effect. Catalytic properties The use of erbium(III) chloride as a catalyst has been demonstrated in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holmium(III) Chloride
Holmium(III) chloride is the inorganic compound with the formula Ho Cl3. It is a common salt but is mainly used in research. It exhibits the same color-changing behavior seen in holmium oxide, being a yellow in natural lighting and a bright pink color in fluorescent lighting. Preparation It forms upon union of the elements, but a more commonly used method involves heating a mixture of holmium(III) oxide Holmium(III) oxide, or holmium oxide is a chemical compound of a rare-earth element holmium and oxygen with the formula Ho2O3. Together with dysprosium(III) oxide (Dy2O3), holmium oxide is one of the most powerfully paramagnetic substances known. ... and ammonium chloride at 200-250 °C:Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. :Ho2O3 + 6 NH4Cl → 2 HoCl3 + 6 NH3 + 2 H2O Structure In the solid state it has the YCl3 layer structure.Wells A.F. (1984) ''Structural Inorganic Chemistry'' 5th edition Oxford ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dysprosium Trichloride
Dysprosium(III) chloride (DyCl3), also known as dysprosium trichloride, is a compound of dysprosium and chlorine. It is a white to yellow solid which rapidly absorbs water on exposure to moist air to form a hexahydrate, DyCl3·6H2O. Simple rapid heating of the hydrate causes partial hydrolysisF. T. Edelmann, P. Poremba, in: ''Synthetic Methods of Organometallic and Inorganic Chemistry'', (W. A. Herrmann, ed.), Vol. 6, Georg Thieme Verlag, Stuttgart, 1997. to an oxychloride, DyOCl. Preparation and reactions DyCl3 is often prepared by the " ammonium chloride route", starting from either Dy2O3 or the hydrated chloride DyCl3·6H2O. These methods produce (NH4)2 yCl5 :10 NH4Cl + Dy2O3 → 2 (NH4)2 yCl5 + 6 NH3 + 3 H2O :DyCl3·6H2O + ''2'' NH4Cl → (NH4)2 yCl5+ 6 H2O The pentachloride decomposes thermally according to the following equation: :(NH4)2 yCl5 → 2 NH4Cl + DyCl3 The thermolysis reaction proceeds via the intermediacy of (NH4) y2Cl7 Treating Dy2O3 with aque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terbium Trichloride
Terbium(III) chloride ( Tb Cl3) is a chemical compound. In the solid state TbCl3 has the YCl3 layer structure. Terbium(III) chloride frequently forms a hexahydrate. Hazards Terbium(III) chloride causes hyperemia of the iris. Conditions/substances to avoid are: heat In thermodynamics, heat is defined as the form of energy crossing the boundary of a thermodynamic system by virtue of a temperature difference across the boundary. A thermodynamic system does not ''contain'' heat. Nevertheless, the term is ..., acids and acid fumes. References Chlorides Lanthanide halides Terbium compounds {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gadolinium Trichloride
Gadolinium(III) chloride, also known as gadolinium trichloride, is GdCl3. It is a colorless, hygroscopic, water-soluble solid. The hexahydrate GdCl3∙6H2O is commonly encountered and is sometimes also called gadolinium trichloride. Gd3+ species are of special interest because the ion has the maximum number of unpaired spins possible, at least for known elements. With seven valence electrons and seven available f-orbitals, all seven electrons are unpaired and symmetrically arranged around the metal. The high magnetism and high symmetry combine to make Gd3+ a useful component in NMR spectroscopy and MRI. Preparation GdCl3 is usually prepared by the " ammonium chloride" route, which involves the initial synthesis of (NH4)2 dCl5 This material can be prepared from the common starting materials at reaction temperatures of 230 °C from gadolinium oxide: ::10 NH4Cl + Gd2O3 → 2 (NH4)2 dCl5 + 6 NH3 + 3 H2O from hydrated gadolinium chloride: ::4 NH4Cl + 2 GdCl3∙6H2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Europium Trichloride
Europium(III) chloride is an inorganic compound with the formula EuCl3. The anhydrous compound is a yellow solid. Being hygroscopic it rapidly absorbs water to form a white crystalline hexahydrate, EuCl3·6H2O, which is colourless. The compound is used in research. Preparation Treating Eu2O3 with aqueous HCl produces hydrated europium chloride (EuCl3·6H2O). This salt cannot be rendered anhydrous by heating. Instead one obtains an oxychloride. Anhydrous EuCl3 is often prepared by the " ammonium chloride route," starting from either Eu2O3 or hydrated europium chloride (EuCl3·6H2O) by heating carefully to 230 °C. These methods produce (NH4)2 uCl5 :10 NH4Cl + Eu2O3 → 2 (NH4)2 uCl5 + 6 NH3 + 3 H2O :EuCl3·6H2O + ''2'' NH4Cl → (NH4)2 uCl5+ 6 H2O The pentachloride decomposes thermally according to the following equation: : (NH4)2 uCl5 → 2 NH4Cl + EuCl3 The thermolysis reaction proceeds via the intermediary of (NH4) u2Cl7 Reactions Europium(III) chloride is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samarium Trichloride
Samarium(III) chloride, also known as samarium trichloride, is an inorganic compound of samarium and chloride. It is a pale yellow salt that rapidly absorbs water to form a hexahydrate, SmCl3.6H2O. The compound has few practical applications but is used in laboratories for research on new compounds of samarium. Structure Like several related chlorides of the lanthanides and actinides, SmCl3 crystallises in the UCl3 motif. The Sm3+ centres are nine-coordinate, occupying trigonal prismatic sites with additional chloride ligands occupying the three square faces. Preparation and reactions SmCl3 is prepared by the " ammonium chloride" route, which involves the initial synthesis of (NH4)2 mCl5 This material can be prepared from the common starting materials at reaction temperatures of 230 °C from samarium oxide: ::10 NH4Cl + Sm2O3 → 2 (NH4)2 mCl5 + 6 NH3 + 3 H2O The pentachloride is then heated to 350-400 °C resulting in evolution of ammonium chloride and leaving ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
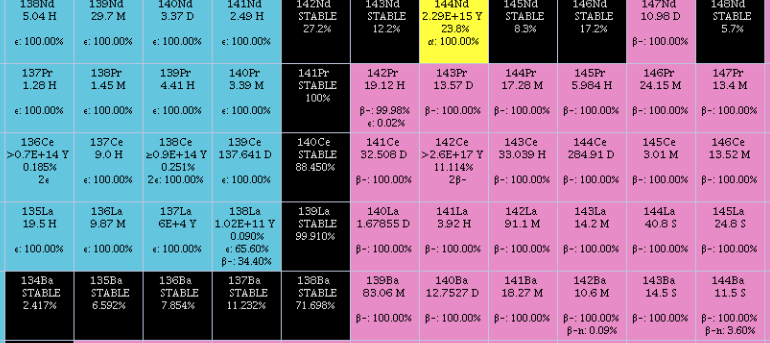

_2.png)