|
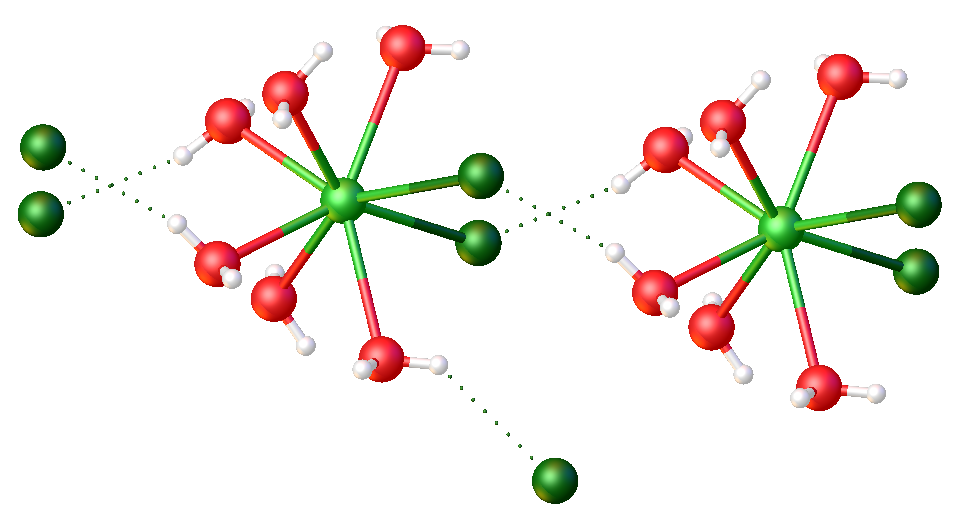
Ytterbium Trichloride
Ytterbium(III) chloride ( Yb Cl3) is an inorganic chemical compound. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides. It is poisonous if injected, and mildly toxic by ingestion. It is an experimental teratogen, known to irritate the skin and eyes. When heated to decomposition it emits toxic fumes of Cl−. History The synthesis of YbCl3 was first reported by Jan Hoogschagen in 1946. It is now a commercially available source of Yb3+ ions and therefore of significant chemical interest. Chemical properties The valence electron configuration of Yb+3 (from YbCl3) is 4''f''135''s''25''p''6, which has crucial implications for the chemical behaviour of Yb+3. Also, the size of Yb+3 governs its catalytic behaviour and biological applications. For example, while both Ce+3 and Yb+3 have a single unpaired ''f'' electron, Ce+3 is much larger than Yb+3 because lanthanides become much smaller with increasing effective nuclear charge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal classes The table below org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicity
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell ( cytotoxicity) or an organ such as the liver (hepatotoxicity). By extension, the word may be metaphorically used to describe toxic effects on larger and more complex groups, such as the family unit or society at large. Sometimes the word is more or less synonymous with poisoning in everyday usage. A central concept of toxicology is that the effects of a toxicant are dose-dependent; even water can lead to water intoxication when taken in too high a dose, whereas for even a very toxic substance such as snake venom there is a dose below which there is no detectable toxic effect. Toxicity is species-specific, making cross-species analysis problematic. Newer paradigms and metrics are evolving to bypas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide Halides
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yttrium, are often collectively known as the rare-earth elements or rare-earth metals. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. There is some dispute on whether lanthanum or lutetium is a d-block element, but lutetium is usually considered so by those who study the matter; it is included due to its chemical similarities with the other 14. All lanthanide elements form trivalent cations, Ln3+, whose chemistry is largely determined by the ionic radius, which decreases steadily from lanthanum to lutetium. These elements are called lanthanides because the elements in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorides
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride salts such as sodium chloride are often very soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Less frequently, the word ''chloride'' may also form part of the "common" name of chemical compounds in which one or more chlorine atoms are covalently bonded. For example, methyl chloride, with the standard name chloromethane (see IUPAC books) is an organic compound with a covalent C−Cl bond in which the chlorine is not an anion. Electronic properties A chloride ion (diameter 167 pm) is much larger than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescein
Fluorescein is an organic compound and dye based on the xanthene tricyclic structural motif, formally belonging to triarylmethine dyes family. It is available as a dark orange/red powder slightly soluble in water and alcohol. It is widely used as a fluorescent tracer for many applications. The color of its aqueous solutions is green by reflection and orange by transmission (its spectral properties are dependent on pH of the solution), as can be noticed in bubble levels, for example, in which fluorescein is added as a colorant to the alcohol filling the tube in order to increase the visibility of the air bubble contained within (thus enhancing the precision of the instrument). More concentrated solutions of fluorescein can even appear red (because under these conditions nearly all incident emission is re-absorbed by the solution). It is on the World Health Organization's List of Essential Medicines. Uses Fluorescein sodium, the sodium salt of fluorescein, is used extensi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inductively Coupled Plasma Mass Spectrometry
Inductively coupled plasma mass spectrometry (ICP-MS) is a type of mass spectrometry that uses an inductively coupled plasma to ionize the sample. It atomizes the sample and creates atomic and small polyatomic ions, which are then detected. It is known and used for its ability to detect metals and several non-metals in liquid samples at very low concentrations. It can detect different isotopes of the same element, which makes it a versatile tool in isotopic labeling. Compared to atomic absorption spectroscopy, ICP-MS has greater speed, precision, and sensitivity. However, compared with other types of mass spectrometry, such as thermal ionization mass spectrometry (TIMS) and glow discharge mass spectrometry (GD-MS), ICP-MS introduces many interfering species: argon from the plasma, component gases of air that leak through the cone orifices, and contamination from glassware and the cones. Components Inductively coupled plasma An inductively coupled plasma is a plasma that is ener ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMR Shift Reagent
A chiral derivatizing agent (CDA) also known as a chiral resolving reagent, is a chiral auxiliary used to convert a mixture of enantiomers into diastereomers in order to analyze the quantities of each enantiomer present within the mix. Analysis can be conducted by spectroscopy or by chromatography. The use of chiral derivatizing agents has declined with the popularization of chiral HPLC. Besides analysis, chiral derivatization is also used for chiral resolution, the actual physical separation of the enantiomers. History Since NMR spectroscopy has been available to chemists, there have been numerous studies on the applications of this technique. One of these noted the difference in the chemical shift (i.e. the distance between the peaks) of two diastereomers. Conversely, two compounds that are enantiomers have the same NMR spectral properties. It was reasoned that if a mix of enantiomers could be converted into a mix of diastereomers by bonding them to another chemical that was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudocontact Shift
Paramagnetic nuclear magnetic resonance spectroscopy refers to nuclear magnetic resonance (NMR) spectroscopy of paramagnetic compounds. Although most NMR measurements are conducted on diamagnetic compounds, paramagnetic samples are also amenable to analysis and give rise to special effects indicated by a wide chemical shift range and broadened signals. Paramagnetism diminishes the resolution of an NMR spectrum to the extent that coupling is rarely resolved. Nonetheless spectra of paramagnetic compounds provide insight into the bonding and structure of the sample. For example, the broadening of signals is compensated in part by the wide chemical shift range (often 200 ppm in 1H NMR). Since paramagnetism leads to shorter relaxation times (T1), the rate of spectral acquisition can be high. : Chemical shifts in diamagnetic compounds are described using the Ramsey equation, which describes so-called diamagnetic and paramagnetic contributions. In this equation, paramagnetic ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanide Chloride
Lanthanide chlorides are a group of chemical compounds that can form between a lanthanide element (from lanthanum to lutetium) and chlorine. The lanthanides in these compounds are usually in the +2 and +3 oxidation states, although compounds with lanthanides in lower oxidation states exist. Lanthanide dichlorides Divalent chlorides are formed by neodymium, samarium, europium, dysprosium, thulium and ytterbium. They can be prepared by reducing the trivalent chloride with lithium metal/naphthalene in tetrahydrofuran: : LnCl3 + Li → LnCl2 + LiCl (Ln=Nd,Sm,Eu) Reducing the chloride with the metal or hydrogen is also possible: : 2 LnCl3 + Ln → 3 LnCl2 (Ln=Nd,Sm,Eu?,Dy,Tm,Yb) : 2 LnCl3 + H2 → 2 LnCl2 + 2 HCl (Ln=Nd,Sm,Eu,Dy,Tm,Yb) Lanthanide trichlorides The lanthanide trichlorides can generally be prepared by dissolving the oxide or carbonate with hydrochloric acid. They are produced commercially by carbothermic reaction of the oxide. To produce the anhydrous forms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Chloride Route
Lanthanide trichlorides are a family of inorganic compound with the formula Ln Cl3, where Ln stands for a lanthanide metal. The trichlorides are standard reagents in applied and academic chemistry of the lanthanides. They exist as anhydrous solids and as hydrates. Properties The anhydrous solids have melting points range from ca. 582 (Tb) - 925 °C (Lu). They are generally pale colored, often white. As coordination polymers, they only dissolve in donor solvents, including water. Preparation The lanthanide oxides and carbonates dissolve in hydrochloric acid to give chloride salt of the hydrated cations: :M2O3 + 6HCl + n H2O → 2 n(H2O)nl3 Industrial routes Anhydrous trichlorides are produced commercially by carbothermic reaction of the oxide: :M2O3 + 3Cl2 + 3C → 2MCl3 + 3CO Ammonium chloride route The ammonium chloride route refers to a general procedure to produce anhydrous lanthanide chlorides. The method has the advantages of being general for the 14 la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical properties, such as whether or not it has a dipole moment, as well as its allowed spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward-Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |