|
Lipid-gated Ion Channels
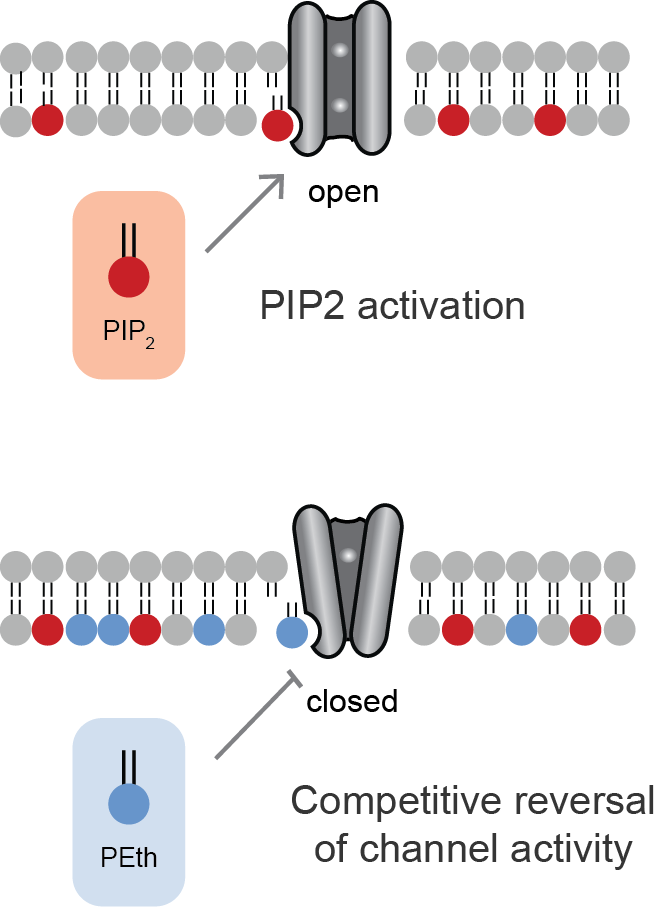
Lipid-gated ion channels are a class of ion channels whose conductance of ions through the membrane depends directly on lipids. Classically the lipids are membrane resident anionic signaling lipids that bind to the transmembrane domain on the inner leaflet of the plasma membrane with properties of a classic ligand. Other classes of lipid-gated channels include the mechanosensitive ion channels that respond to lipid tension, thickness, and hydrophobic mismatch. A lipid ligand differs from a lipid cofactor in that a ligand derives its function by dissociating from the channel while a cofactor typically derives its function by remaining bound. PIP2-gated channels Phosphatidylinositol 4,5-bisphosphate (PIP2) was the first and remains the best studied lipid to gate ion channels. PIP2 is a cell membrane lipid, and its role in gating ion channels represents a novel role for the molecule. Kir channels: PIP2 binds to and directly activates inwardly rectifying potassium channels (Kir) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inward-rectifier Potassium Ion Channel
Inward-rectifier potassium channels (Kir, IRK) are a specific Lipid-gated_ion_channels, lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (Phosphatidylinositol 4,5-bisphosphate, PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane domain, transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium, potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian, 2nd Baron Adrian, Richard Adrian and Alan Lloyd Hodgkin, Alan Hodgkin in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand (biochemistry)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KvLQT1
Kv7.1 (KvLQT1) is a potassium channel protein whose primary subunit in humans is encoded by the ''KCNQ1'' gene. Kv7.1 is a voltage and lipid-gated potassium channel present in the cell membranes of cardiac tissue and in inner ear neurons among other tissues. In the cardiac cells, Kv7.1 mediates the IKs (or slow delayed rectifying K+) current that contributes to the repolarization of the cell, terminating the cardiac action potential and thereby the heart's contraction. It is a member of the KCNQ family of potassium channels. Structure KvLQT1 is made of six membrane-spanning domains S1-S6, two intracellular domains, and a pore loop. The KvLQT1 channel is made of four KCNQ1 subunits, which form the actual ion channel. Function This gene encodes a protein for a voltage-gated potassium channel required for the repolarization phase of the cardiac action potential. The gene product can form heteromultimers with two other potassium channel proteins, KCNE1 and KCN ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidylglycerol
Phosphatidylglycerol is a glycerophospholipid found in pulmonary surfactant and in the plasma membrane where it directly activates lipid-gated ion channels. The general structure of phosphatidylglycerol consists of a L-glycerol 3-phosphate backbone ester-bonded to either saturated or unsaturated fatty acids on carbons 1 and 2. The head group substituent glycerol is bonded through a phosphomonoester. It is the precursor of surfactant and its presence (>0.3) in the amniotic fluid of the newborn indicates fetal lung maturity. Approximately 98% of alveolar wall surface area is due to the presence of type I cells, with type II cells producing pulmonary surfactant covering around 2% of the alveolar walls. Once surfactant is secreted by the type II cells, it must be spread over the remaining type I cellular surface area. Phosphatidylglycerol is thought to be important in spreading of surfactant over the Type I cellular surface area. The major surfactant deficiency in premature infants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KCNK2
Potassium channel subfamily K member 2, also known as TREK-1, is a protein that in humans is encoded by the ''KCNK2'' gene. This gene encodes K2P2.1, a lipid-gated ion channel belonging to the two-pore-domain background potassium channel protein family. This type of potassium channel is formed by two homodimers that create a channel that releases potassium out of the cell to control resting membrane potential. The channel is opened by anionic lipid, certain anesthetics, membrane stretching, intracellular acidosis, and heat. Three transcript variants encoding different isoforms have been found for this gene. Function in neurons TREK-1 is part of the subfamily of mechano-gated potassium channels that are present in mammalian neurons. They can be gated in both chemical and physical ways and can be opened via both physical stimuli and chemical stimuli. TREK-1 channels are found in a variety of tissues, but are particularly abundant in the brain and heart and are seen in various type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inotropic
An inotrope is an agent that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction. The term ''inotropic state'' is most commonly used in reference to various drugs that affect the strength of contraction of heart muscle. However, it can also refer to pathological conditions. For example, enlarged heart muscle can increase inotropic state, whereas dead heart muscle can decrease it. Medical uses Both positive and negative inotropes are used in the management of various cardiovascular conditions. The choice of agent depends largely on specific pharmacological effects of individual agents with respect to the condition. One of the most important factors affecting inotropic state is the level of calcium in the cytoplasm of the muscle cell. Positive inotropes usually increase this level, while negative inotropes decrease it. However, not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinic Acetylcholine Receptor
Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system. The nicotinic receptors are considered choline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small-conductance Mechanosensitive Channel
Members of the Small Conductance Mechanosensitive Ion Channel (MscS) Family provide protection against hypo-osmotic shock in bacteria, responding both to stretching of the cell membrane and to membrane depolarization. In eukaryotes, they fulfill a multitude of important functions in addition to osmoregulation. They are present in the membranes of organisms from the three domains of life: bacteria, archaea, fungi and plants. Structure There are two families of mechanosensitive (MS) channels: large-conductance MS channels (MscL) and small-conductance MS channels (MscS or YGGB). The MscS family is much larger and more variable in size and sequence than the MscL family. MscS family homologues vary in length between 248 and 1120 amino acyl residues and in topology, but the homologous region that is shared by most of them is only 200-250 residues long, exhibiting 4-5 transmembrane regions (TMSs). Much of the diversity in MscS proteins occurs in the number of TMSs, which ranges fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Large-conductance Mechanosensitive Channel
The Large Conductance Mechanosensitive Ion Channel (MscL) FamilyTC# 1.A.22 consists of pore-forming membrane proteins that are responsible for translating physical forces applied to cell membranes into electrophysiological activities. MscL has a relatively large conductance, 3 nS, making it permeable to ions, water, and small proteins when opened. MscL acts as stretch-activated osmotic release valve in response to osmotic shock. History MscL was first discovered on the surface of giant ''Escherichia coli'' spheroplasts using patch-clamp technique. Subsequently, the ''Escherichia coli'' MscL (Ec-MscL) gene was cloned in 1994. Following the cloning of MscL, the crystal structure of '' Mycobacterium tuberculosis'' MscL (Tb-MscL), was obtained in its closed conformation. In addition, the crystal structure of ''Staphylococcus aureus'' MscL (Sa-MscL) and Ec-MscL have been determined using X-ray crystallography and molecular model respectively. However, some evidence suggests that the S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanosensitive Channels
Mechanosensitive channels, mechanosensitive ion channels or stretch-gated ion channels (not to be confused with mechanoreceptors). They are present in the membranes of organisms from the three domains of life: bacteria, archaea, and eukarya. They are the sensors for a number of systems including the senses of touch, hearing and balance, as well as participating in cardiovascular regulation and osmotic homeostasis (e.g. thirst). The channels vary in selectivity for the permeating ions from nonselective between anions and cations in bacteria, to cation selective allowing passage Ca2+, K+ and Na+ in eukaryotes, and highly selective K+ channels in bacteria and eukaryotes. All organisms, and apparently all cell types, sense and respond to mechanical stimuli. MSCs function as mechanotransducers capable of generating both electrical and ion flux signals as a response to external or internal stimuli. Under extreme turgor in bacteria, non selective MSCs such as MSCL and MSCS serve as safe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidic Acid
Phosphatidic acids are anionic phospholipids important to cell signaling and direct activation of lipid-gated ion channels. Hydrolysis of phosphatidic acid gives rise to one molecule each of glycerol and phosphoric acid and two molecules of fatty acids. They constitute about 0.25% of phospholipids in the bilayer. Structure Phosphatidic acid consists of a glycerol backbone, with, in general, a saturated fatty acid bonded to carbon-1, an unsaturated fatty acid bonded to carbon-2, and a phosphate group bonded to carbon-3. Formation and degradation Besides de novo synthesis, PA can be formed in three ways: * By phospholipase D (PLD), via the hydrolysis of the P-O bond of phosphatidylcholine (PC) to produce PA and choline. * By the phosphorylation of diacylglycerol (DAG) by DAG kinase (DAGK). * By the acylation of lysophosphatidic acid by lysoPA-acyltransferase (LPAAT); this is the most common pathway.Devlin, T. M. 2004. ''Bioquímica'', 4ª edición. Reverté, Barcelona. The glycer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |