|
Isotopes Of Caesium
Caesium (55Cs) has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. The atomic masses of these isotopes range from 112 to 151. Only one isotope, 133Cs, is stable. The longest-lived radioisotopes are 135Cs with a half-life of 2.3 million years, with a half-life of 30.1671 years and 134Cs with a half-life of 2.0652 years. All other isotopes have half-lives less than 2 weeks, most under an hour. Beginning in 1945 with the commencement of nuclear testing, caesium radioisotopes were released into the atmosphere where caesium is absorbed readily into solution and is returned to the surface of the earth as a component of radioactive fallout. Once caesium enters the ground water, it is deposited on soil surfaces and removed from the landscape primarily by particle transport. As a result, the input function of these isotopes can be estimated as a function of time. List of isotopes , - , rowspan=2, 112Cs , rowspan=2 style="text-a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IsoRay
Isoray Inc. (Isoray) is a national isotope-based medical company and the sole producer of Cesium brachytherapy sources, which are expanding brachytherapy treatments for difficult to treat cancers. Isoray is a registered manufacturer with the FDA The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food ... and holds multiple 510(k) clearances for brachytherapy devices. The brachytherapy isotopes are sold under the brandname Blu. The company went public in 2005. Isoray’s corporate headquarters is located at 350 Hills Street, Suite 106, Richland WA 99354. References ;Notes {{reflist Companies listed on NYSE American Health care companies established in 2004 2005 initial public offerings ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoric and reacts with water even at . It is the least electronegative element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. The element has 40 known isotopes, making it, along with barium and mercury, one of the elements with the most isotopes. Caesium-137, a fission product, is extracted from waste produced by nuclear reactors. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the newly developed method of flame spectroscopy. The first small-scale applications for caesium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium-137
Caesium-137 (), cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nuclear weapons. Trace quantities also originate from spontaneous fission of uranium-238. It is among the most problematic of the short-to-medium-lifetime fission products. Caesium-137 has a relatively low boiling point of and is volatilized easily when released suddenly at high temperature, as in the case of the Chernobyl nuclear accident and with atomic explosions, and can travel very long distances in the air. After being deposited onto the soil as radioactive fallout, it moves and spreads easily in the environment because of the high water solubility of caesium's most common chemical compounds, which are salts. Caesium-137 was discovered by Glenn T. Seaborg and Margaret Melhase. Decay Caesium-137 has a half-life of about 30.05 years. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Barium
Naturally occurring barium (56Ba) is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, identified as being unstable by geochemical means (from analysis of the presence of its daughter xenon-130 in rocks) in 2001. This nuclide decays by double electron capture (absorbing two electrons and emitting two neutrinos), with a half-life of (0.5–2.7)×1021 years (about 1011 times the age of the universe). There are a total of thirty-three known radioisotopes in addition to 130Ba. The longest-lived of these is 133Ba, which has a half-life of 10.51 years. All other radioisotopes have half-lives shorter than two weeks. The longest-lived isomer is 133mBa, which has a half-life of 38.9 hours. The shorter-lived 137mBa (half-life 2.55 minutes) arises as the decay product of the common fission product caesium-137. Barium-114 is predicted to undergo cluster decay, emitting a nucleus of stable 12C to produce 102Sn. However this decay is not yet obse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Weapons
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions (thermonuclear bomb), producing a nuclear explosion. Both bomb types release large quantities of energy from relatively small amounts of matter. The first test of a fission ("atomic") bomb released an amount of energy approximately equal to . The first thermonuclear ("hydrogen") bomb test released energy approximately equal to . Nuclear bombs have had yields between 10 tons TNT (the W54) and 50 megatons for the Tsar Bomba (see TNT equivalent). A thermonuclear weapon weighing as little as can release energy equal to more than . A nuclear device no larger than a conventional bomb can devastate an entire city by blast, fire, and radiation. Since they are weapons of mass destruction, the proliferation of nuclear weapons is a focus of international relations policy. Nuclear weapons have been deployed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclides
A nuclide (or nucleide, from nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state. The word ''nuclide'' was coined by Truman P. Kohman in 1947. Kohman defined ''nuclide'' as a "species of atom characterized by the constitution of its nucleus" containing a certain number of neutrons and protons. The term thus originally focused on the nucleus. Nuclides vs isotopes A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, while the isotope concept (grouping all atoms of each element) emphasizes chemical over nuclear. The neutron number has large effects on nuclear properties, but its effect on chemical reactions is negligible for most elements. Even in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barn (unit)
A barn (symbol: b) is a metric unit of area equal to (100 fm2). Originally used in nuclear physics for expressing the cross sectional area of nuclei and nuclear reactions, today it is also used in all fields of high-energy physics to express the cross sections of any scattering process, and is best understood as a measure of the probability of interaction between small particles. A barn is approximately the cross-sectional area of a uranium nucleus. The barn is also the unit of area used in nuclear quadrupole resonance and nuclear magnetic resonance to quantify the interaction of a nucleus with an electric field gradient. While the barn never was an SI unit, the SI standards body acknowledged it in the 8th SI Brochure (superseded in 2019) due to its use in particle physics. Etymology During Manhattan Project research on the atomic bomb during World War II, American physicists at Purdue University needed a secretive name for a unit with which to quantify the cross-secti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
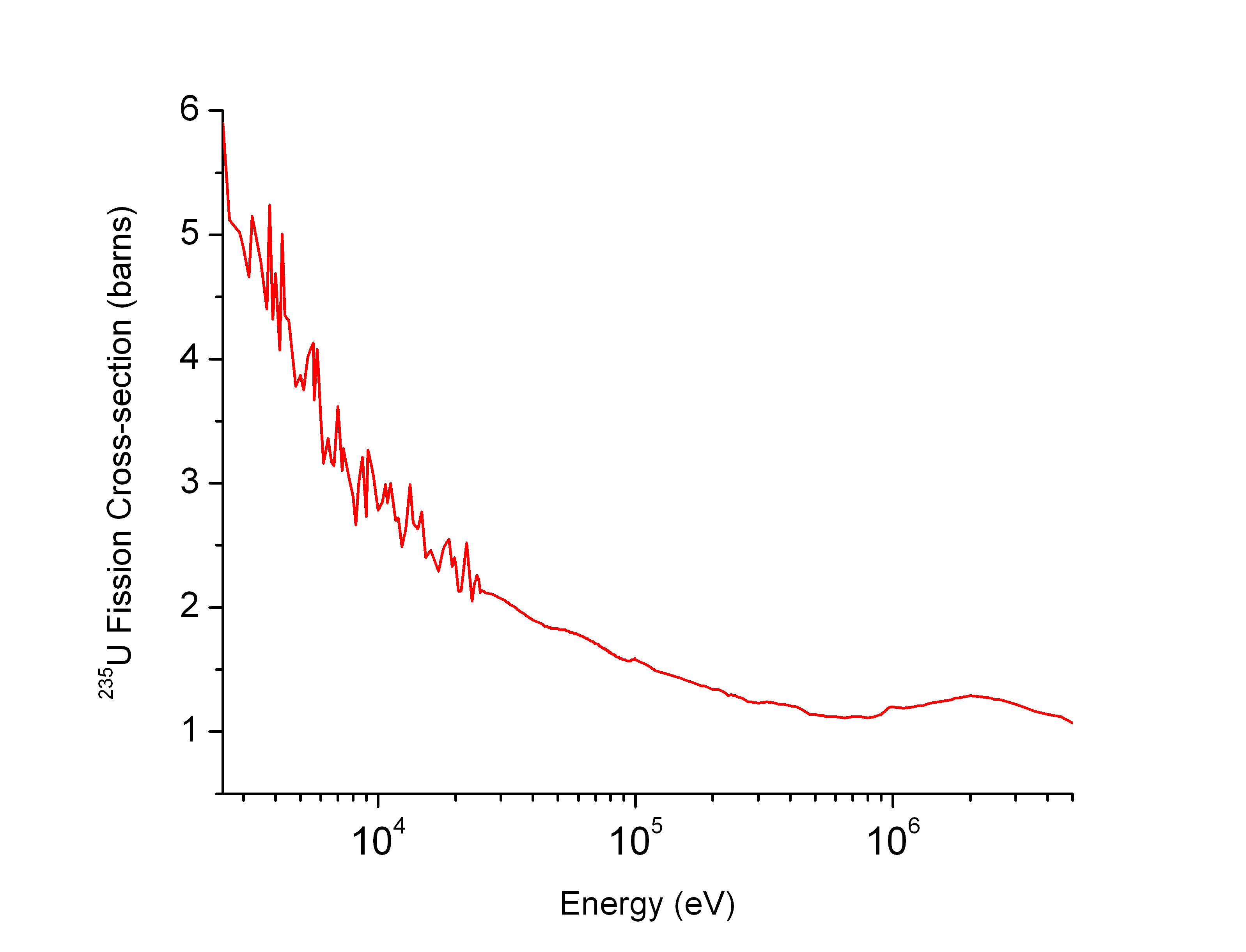
Neutron Cross-section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically. Neutron capture plays a significant role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid process (r-process) or a slow process (s-process). Nuclei of masses greater than 56 cannot be formed by thermonuclear reactions (i.e., by nuclear fusion) but can be formed by neutron capture. Neutron capture on protons yields a line at 2.223 MeV predicted and commonly observed in solar flares. Neutron capture at small neutron flux At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons (n), the isotope 198Au is formed in a highly excited state, and quickly deca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and gamma radiation, even though this radiation is not produced directly by the fission event itself. The produced radionuclides have varyi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caesium Standard
The caesium standard is a primary frequency standard in which the Absorption (electromagnetic radiation), photon absorption by transitions between the two hyperfine level, hyperfine ground states of caesium-133 atoms is used to control the output frequency. The first caesium clock was built by Louis Essen in 1955 at the National Physical Laboratory, UK, National Physical Laboratory in the UK. and promoted worldwide by Gernot M. R. Winkler of the United States Naval Observatory. Caesium atomic clocks are one of the most accurate time and frequency standards, and serve as the primary standard for the definition of the second in the International System of Units (SI) (the modern form of the metric system). By definition, radiation produced by the transition between the two hyperfine ground states of caesium (in the absence of external influences such as the Earth's magnetic field) has a frequency, , of exactly . That value was chosen so that the caesium second equalled, to the limit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SI Base Unit
The SI base units are the standard units of measurement defined by the International System of Units (SI) for the seven base quantities of what is now known as the International System of Quantities: they are notably a basic set from which all other SI units can be derived. The units and their physical quantities are the second for time, the metre (sometimes spelled meter) for length or distance, the kilogram for mass, the ampere for electric current, the kelvin for thermodynamic temperature, the mole for amount of substance, and the candela for luminous intensity. The SI base units are a fundamental part of modern metrology, and thus part of the foundation of modern science and technology. The SI base units form a set of mutually independent dimensions as required by dimensional analysis commonly employed in science and technology. The names and symbols of SI base units are written in lowercase, except the symbols of those named after a person, which are written with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |