|
Isooctane
2,2,4-Trimethylpentane, also known as isooctane or iso-octane, is an organic compound with the formula (CH3)3CCH2CH(CH3)2. It is one of several isomers of octane (C8H18). This particular isomer is the standard 100 point on the octane rating scale (the zero point is ''n''-heptane). It is an important component of gasoline, frequently used in relatively large proportions (around 10%) to increase the knock resistance of fuel. Strictly speaking, if the standard meaning of ‘iso’ is followed, the name ''isooctane'' should be reserved for the isomer 2-methylheptane. However, 2,2,4-trimethylpentane is by far the most important isomer of octane and historically it has been assigned this name. Production Isooctane is produced on a massive scale in the petroleum industry by alkylation of isobutene with isobutane. This process is conducted in alkylation units in the presence of acid catalysts. It can also be produced from isobutylene by dimerization using an Amberlyst catalyst to pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2-Dimethylbutane
2,2-Dimethylbutane, trivially known as neohexane, is an organic compound with formula C6H14 or (H3C-)3-C-CH2-CH3. It is therefore an alkane, indeed the most compact and branched of the hexane isomers — the only one with a quaternary carbon and a butane (C4) backbone. Synthesis 2,2-Dimethylbutane can be synthesised by the hydroisomerisation of 2,3-dimethylbutane using an acid catalyst. It can also be synthesised by isomerization of ''n''-pentane in the presence of a catalyst containing combinations of one or more of palladium, platinum, rhodium and rhenium on a matrix of zeolite, alumina, silicon dioxide or other materials. Such reactions create a mixture of final products including isopentane, ''n''-hexane, 3-methylpentane, 2-methylpentane, 2,3-dimethylbutane and 2,2-dimethylbutane. Since the composition of the final mixture is temperature dependant the desired final component can be obtained choice of catalyst and by combinations of temperature control and distillations. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-methylheptane
2-Methylheptane is a branched alkane isomeric to octane. Its structural formula The structural formula of a chemical compound is a graphic representation of the molecular structure (determined by structural chemistry methods), showing how the atoms are possibly arranged in the real three-dimensional space. The chemical bond ... is (CH3)2CH(CH2)4CH3. References External links *Chemical and physical properties table {{DEFAULTSORT:Methylheptane, 2- Alkanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graham Edgar
Graham and Graeme may refer to: People * Graham (given name), an English-language given name * Graham (surname), an English-language surname * Graeme (surname), an English-language surname * Graham (musician) (born 1979), Burmese singer * Clan Graham, a Scottish clan * Graham baronets Fictional characters * Graham Aker, in the anime ''Gundam 00'' * Project Graham, what a human would look like to survive a car crash Places Canada * Graham, Sudbury District, Ontario * Graham Island, part of the Charlotte Island group in British Columbia * Graham Island (Nunavut), Arctic island in Nunavut United States * Graham, Alabama * Graham, Arizona * Graham, Florida * Graham, Georgia * Graham, Daviess County, Indiana * Graham, Fountain County, Indiana * Graham, Kentucky * Graham, Missouri * Graham, North Carolina * Graham, Oklahoma * Graham, Texas * Graham, Washington Elsewhere * Graham Land, Antarctica * Graham Island (Mediterranean Sea), British name for a submerged volcanic i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
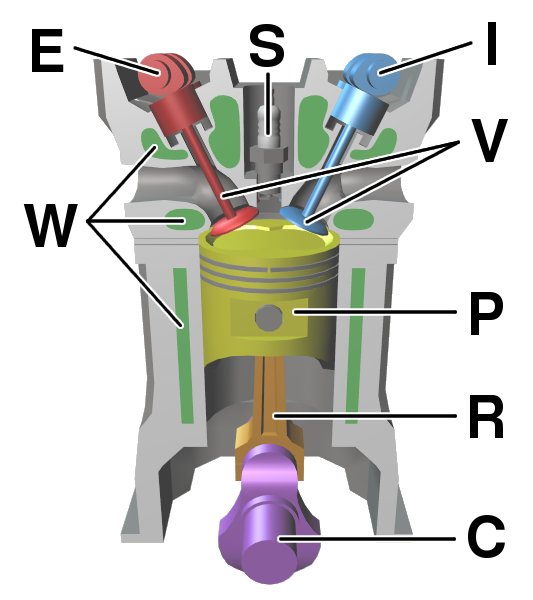
Internal Combustion Engine
An internal combustion engine (ICE or IC engine) is a heat engine in which the combustion of a fuel occurs with an oxidizer (usually air) in a combustion chamber that is an integral part of the working fluid flow circuit. In an internal combustion engine, the expansion of the high- temperature and high- pressure gases produced by combustion applies direct force to some component of the engine. The force is typically applied to pistons (piston engine), turbine blades ( gas turbine), a rotor (Wankel engine), or a nozzle (jet engine). This force moves the component over a distance, transforming chemical energy into kinetic energy which is used to propel, move or power whatever the engine is attached to. This replaced the external combustion engine for applications where the weight or size of an engine was more important. The first commercially successful internal combustion engine was created by Étienne Lenoir around 1860, and the first modern internal combustion engine, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces double and triple bonds in hydrocarbons. Process Hydrogenation has three components, the unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same catalysts and conditions that are used for hydrogenation reactions can also lead to isomerization of the alkenes f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some sta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amberlyst
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous, providing a large surface area on and inside them where the trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin. Most commercial resins are made of polystyrene sulfonate.François Dardel and Thomas V. Arden "Ion Exchangers" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. . Ion-exchange resins are widely used in different separation, purification, and decontamination processes. The most common examples are water softening and water purification. In many cases ion-exchange resins were introduced in such proces ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () (''di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutylene
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula . It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value. Production Polymer and chemical grade isobutylene is typically obtained by dehydrating tertiary butyl alcohol (TBA) or catalytic dehydrogenation of isobutane (Catofin or similar processes).. Gasoline additives methyl tert-butyl ether (MTBE) and ethyl tert-butyl ether (ETBE), respectively, are produced by reacting methanol or ethanol with isobutylene contained in butene streams from olefin steam crackers or refineries, or with isobutylene from dehydrated TBA. Isobutylene is not isolated from the olefin or refinery butene stream before the reaction, as separating the ethers from the remaining butenes is simpler. Isobutylene can also be produced in high purities by "back-cracking" MTBE or ETBE at high temperatures and then separating the isobuty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Route2,2,4-Me3pentane
Route or routes may refer to: * Route (gridiron football), a path run by a wide receiver * route (command), a program used to configure the routing table * Route, County Antrim, an area in Northern Ireland * ''The Route'', a 2013 Ugandan film * Routes, Seine-Maritime, a commune in Seine-Maritime, France * ''Routes'' (video game), 2003 video game See also * Acronyms and abbreviations in avionics * Air route or airway * GPS route, a series of one or more GPS waypoints * Path (other) * Rout, a disorderly retreat of military units from the field of battle * Route number or road number * Router (other) * Router (woodworking) * Routing (other) * Routing table * Scenic route, a thoroughfare designated as scenic based on the scenery through which it passes * Trade route A trade route is a logistical network identified as a series of pathways and stoppages used for the commercial transport of cargo. The term can also be used to refer to trade over bodies o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Catalyst
In acid catalysis and base catalysis, a chemical reaction is catalyzed by an acid or a base. By Brønsted–Lowry acid–base theory, the acid is the proton (hydrogen ion, H+) donor and the base is the proton acceptor. Typical reactions catalyzed by proton transfer are esterifications and aldol reactions. In these reactions, the conjugate acid of the carbonyl group is a better electrophile than the neutral carbonyl group itself. Depending on the chemical species that act as the acid or base, catalytic mechanisms can be classified as either specific catalysis and general catalysis. Many enzymes operate by general catalysis. Applications and examples Brønsted acids Acid catalysis is mainly used for organic chemical reactions. Many acids can function as sources for the protons. Acid used for acid catalysis include hydrofluoric acid (in the alkylation process), phosphoric acid, toluenesulfonic acid, polystyrene sulfonate, heteropoly acids, zeolites. Strong acids catalyze the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation Unit
An alkylation unit (alky) is one of the conversion processes used in Oil industry, petroleum refineries. It is used to convert isobutane and low-molecular-weight alkenes (primarily a mixture of propene and butene) into alkylate, a high octane gasoline component. The process occurs in the presence of an acid such as sulfuric acid (H2SO4) or hydrofluoric acid (HF) as catalyst. Depending on the acid used, the unit is called a sulfuric acid alkylation unit (SAAU) or hydrofluoric acid alkylation unit (HFAU). In short, the alky produces a high-quality gasoline blending stock by combining two shorter hydrocarbon molecules into one longer chain gasoline-range molecule by mixing isobutane with a light olefin such as propylene or butylene from the refinery's fluid catalytic cracking unit (FCCU) in the presence of an acid catalyst. Since crude oil generally contains only 10-40% of hydrocarbon constituents in the gasoline range, refineries typically use an FCCU to convert high molecular weight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |