|
Ionic Solution
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. El ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conductivity (electrolytic)
Conductivity (or specific conductance) of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is Siemens per meter (S/m). Conductivity measurements are used routinely in many industrial and environmental applications as a fast, inexpensive and reliable way of measuring the ionic content in a solution. For example, the measurement of product conductivity is a typical way to monitor and continuously trend the performance of water purification systems. In many cases, conductivity is linked directly to the total dissolved solids (TDS). High quality deionized water has a conductivity of about 0.05 μS/cm at 25 °C, typical drinking water is in the range of 200–800 μS/cm, while sea water is about 50 mS/cm ncorrect according to source(or 50,000 μS/cm). Conductivity is traditionally determined by connecting the electrolyte in a Wheatstone bridge. Dilute solutions follow Kohlrausch's Laws of concentrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anorexia Nervosa
Anorexia nervosa, often referred to simply as anorexia, is an eating disorder characterized by low weight, food restriction, body image disturbance, fear of gaining weight, and an overpowering desire to be thin. ''Anorexia'' is a term of Greek origin: ''an-'' (ἀν-, prefix denoting negation) and ''orexis'' (ὄρεξις, "appetite"), translating literally to "a loss of appetite"; the adjective ''nervosa'' indicating the functional and non-organic nature of the disorder. ''Anorexia nervosa'' was coined by Gull in 1873 but, despite literal translation, the feeling of hunger is frequently present and the pathological control of this instinct is a source of satisfaction for the patients. Individuals with anorexia nervosa have a fear of being overweight or being seen as such, although they are in fact underweight. The DSM-5 describes this perceptual symptom as "disturbance in the way in which one's body weight or shape is experienced". In research and clinical settings, thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sports Drink
Sports drinks, also known as electrolyte drinks, are functional beverages whose stated purpose is to help Sportsperson, athletes replace water, electrolytes, and energy before, during and especially after training or competition. There are many perceived benefits and questions pertaining to the efficacy of use for sports and fitness performance. At times if used too much, in wrong cases (as most of nutritional items) they may hinder health or performance. The drinks, or some of their ingredients such as sugar, may not be suitable for certain conditions. Categories of sport drinks Sports drinks can be split into three major types: *Tonicity#Isotonicity, Isotonic sport drinks contain similar concentrations of salt and sugar as in the human body. *Tonicity#Hypertonic solution, Hypertonic sport drinks contain a higher concentration of salt and sugar than the human body. *Tonicity#Hypotonicity, Hypotonic sport drinks contain a lower concentration of salt and sugar than the human body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pedialyte
Pedialyte is an oral electrolyte solution manufactured by Abbott Laboratories and marketed for use in children. It was invented by Dr. Gary Cohen of Swampscott, Massachusetts. Description Pedialyte is designed to promote rehydration and electrolyte replacement in ill children. It "meets the requirements of the American Academy of Pediatrics (AAP) Committee on Nutrition to help prevent dehydration in infants and children." Pedialyte is lower in sugars than most sports drinks, containing 100 calories per liter compared to approximately 240 in Gatorade. It contains more sodium (1,035 milligrams per liter vs. 465 mg/L in Gatorade) and potassium (780 milligrams per liter vs. 127 mg/L in Gatorade). Pedialyte does not contain sucrose, because this sugar has the potential to make diarrhea worse by drawing water into the intestine, increasing the risk of dehydration. In its flavored formulations, Pedialyte uses the synthetic sweeteners sucralose and acesulfame potassium. Pedialyte ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Suero Oral
In the United States, Suero Oral® is a brand name of an electrolyte solution used to re-hydrate after working in heat-intensive environments, athletic activity, to treat pediatric vomiting and diarrhea, and as a hangover remedy. The product is similar in formula to other popular pediatric electrolyte beverages such as Pedialyte®. The name originated as a reference to suero casero, a whey-based home remedy (also known simply as suero) given to children in parts of South and Central America, the Caribbean, and other Spanish speaking areas. These homemade solutions are common to many households and used to combat dehydration caused by illness, work in extreme heat, or by certain diseases. Oftentimes, in these regions, these homemade solutions are referred to casually as ''suero casero'' (homemade serum), or ''sueros'', but this usage has not extended to the United States. In the United States, the product Suero Oral® contains a blend of water with sugars, flavoring agents (e.g. l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
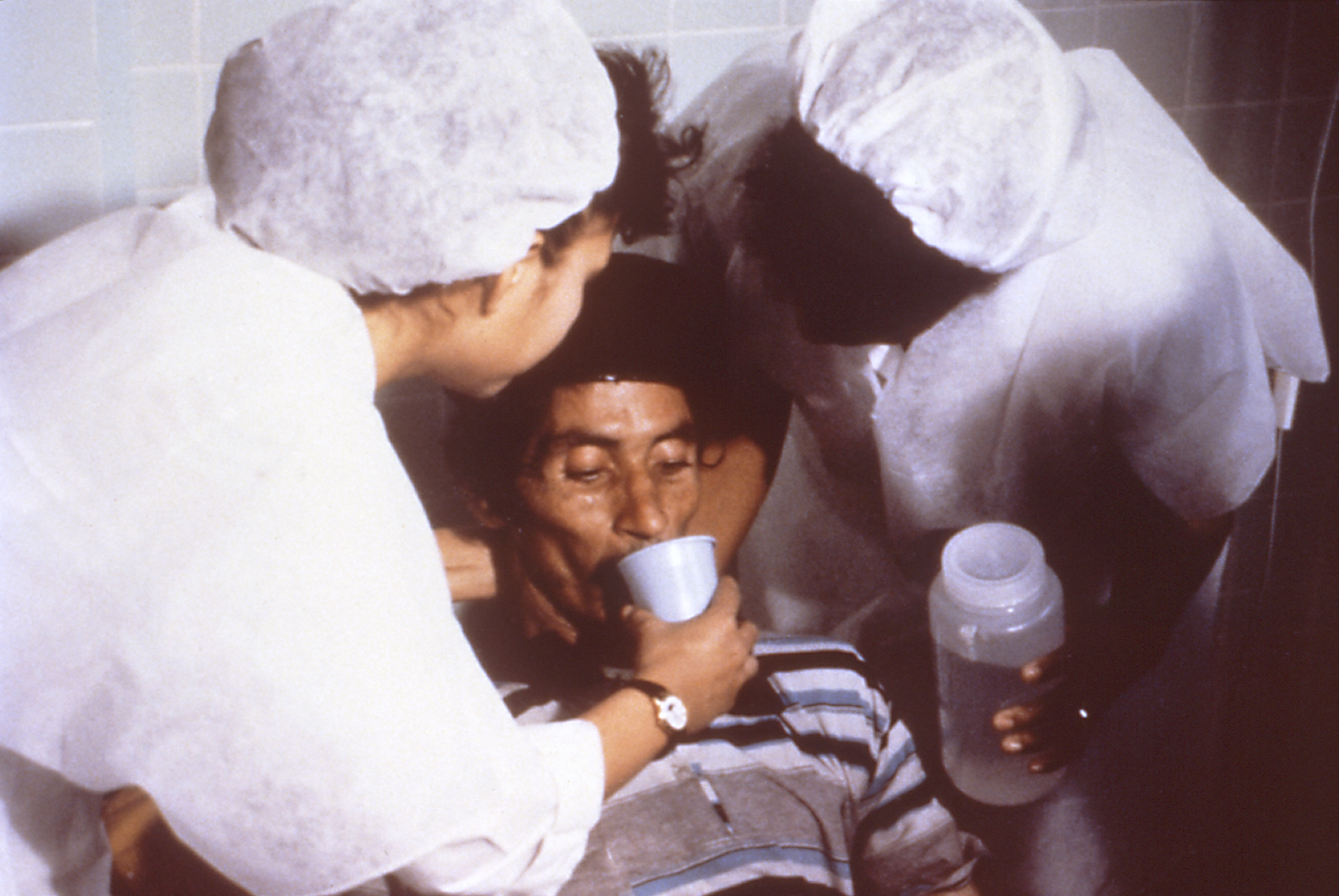
Oral Rehydration Therapy
Oral rehydration therapy (ORT) is a type of fluid replacement used to prevent and treat dehydration, especially due to diarrhea. It involves drinking water with modest amounts of sugar and salts, specifically sodium and potassium. Oral rehydration therapy can also be given by a nasogastric tube. Therapy should routinely include the use of zinc supplements. Use of oral rehydration therapy has been estimated to decrease the risk of death from diarrhea by up to 93%. Side effects may include vomiting, high blood sodium, or high blood potassium. If vomiting occurs, it is recommended that use be paused for 10 minutes and then gradually restarted. The recommended formulation includes sodium chloride, sodium citrate, potassium chloride, and glucose. Glucose may be replaced by sucrose and sodium citrate may be replaced by sodium bicarbonate, if not available. It works as glucose increases the uptake of sodium and thus water by the intestines. A number of other formulations are also ava ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diarrhea
Diarrhea, also spelled diarrhoea, is the condition of having at least three loose, liquid, or watery bowel movements each day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin with loss of the normal stretchiness of the skin and irritable behaviour. This can progress to decreased urination, loss of skin color, a fast heart rate, and a decrease in responsiveness as it becomes more severe. Loose but non-watery stools in babies who are exclusively breastfed, however, are normal. The most common cause is an infection of the intestines due to either a virus, bacterium, or parasite—a condition also known as gastroenteritis. These infections are often acquired from food or water that has been contaminated by feces, or directly from another person who is infected. The three types of diarrhea are: short duration watery diarrhea, short duration bloody diarrhea, and persistent diarrhea (lasting more than two weeks, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vomiting
Vomiting (also known as emesis and throwing up) is the involuntary, forceful expulsion of the contents of one's stomach through the mouth and sometimes the Human nose, nose. Vomiting can be the result of ailments like Food-poisoning, food poisoning, gastroenteritis, pregnancy, motion sickness, or hangover; or it can be an after effect of diseases such as brain tumors, elevated intracranial pressure, or overexposure to ionizing radiation. The feeling that one is about to vomit is called nausea; it often precedes, but does not always lead to vomiting. Impairment due to Alcoholic drink, alcohol or anesthesia can cause inhalation of vomit, leading to suffocation. In severe cases, where dehydration develops, intravenous fluid may be required. Antiemetics are sometimes necessary to suppress nausea and vomiting. Self-induced vomiting can be a component of an eating disorder such as bulimia nervosa, bulimia, and is itself now classified as an eating disorder on its own, purging di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Rehydration Therapy
Oral rehydration therapy (ORT) is a type of fluid replacement used to prevent and treat dehydration, especially due to diarrhea. It involves drinking water with modest amounts of sugar and salts, specifically sodium and potassium. Oral rehydration therapy can also be given by a nasogastric tube. Therapy should routinely include the use of zinc supplements. Use of oral rehydration therapy has been estimated to decrease the risk of death from diarrhea by up to 93%. Side effects may include vomiting, high blood sodium, or high blood potassium. If vomiting occurs, it is recommended that use be paused for 10 minutes and then gradually restarted. The recommended formulation includes sodium chloride, sodium citrate, potassium chloride, and glucose. Glucose may be replaced by sucrose and sodium citrate may be replaced by sodium bicarbonate, if not available. It works as glucose increases the uptake of sodium and thus water by the intestines. A number of other formulations are also ava ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the triv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)