|
Intra-molecular
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule. Examples * intramolecular hydride transfer (transfer of a hydride ion from one part to another within the same molecule) * intramolecular hydrogen bond (a hydrogen bond formed between two functional groups of the same molecule) *cyclization of ω-haloalkylamines and alcohols to form the corresponding saturated nitrogen and oxygen heterocycles, respectively (an SN2 reaction within the same molecule) In intramolecular organic reactions, two reaction sites are contained within a single molecule. This creates a very high effective concentration (resulting in high reaction rates), and, therefore, many intramolecular reactions that would not occur as an intermolecular reaction between two compounds take place. Examples of intramolecular reactions are the Smiles rearrangement, the Dieckmann condensation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a Chemical reaction, reaction with other Chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-beta Unsaturated Carbonyl Compounds
Alphabeta is an Israeli musical group. Alphabeta or Alpha Beta may also refer to: *The Greek alphabet, from ''Alpha'' (Αα) and ''Beta'' (Ββ), the first two letters *Alpha Beta, a former chain of Californian supermarkets *Alpha and beta anomers (chemistry) *Alpha–beta pruning, a type of search algorithm *Alpha-beta transformation, a mathematical transformation in electrical engineering *Alpha-beta unsaturated carbonyl compounds, a class of organic compounds *Alpha beta filter, a predictive filter *Alpha (finance) and Beta (finance), two measures characterizing the return of an investment portfolio *The Alpha Betas, a fraternity in the ''Revenge of the Nerds ''Revenge of the Nerds'' is a 1984 American comedy film directed by Jeff Kanew and starring Robert Carradine, Anthony Edwards, Ted McGinley, and Bernie Casey. The film's plot chronicles a group of nerds at the fictional Adams College trying ...'' film series *''Alpha Betas,'' an animated webseries created by Chris B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
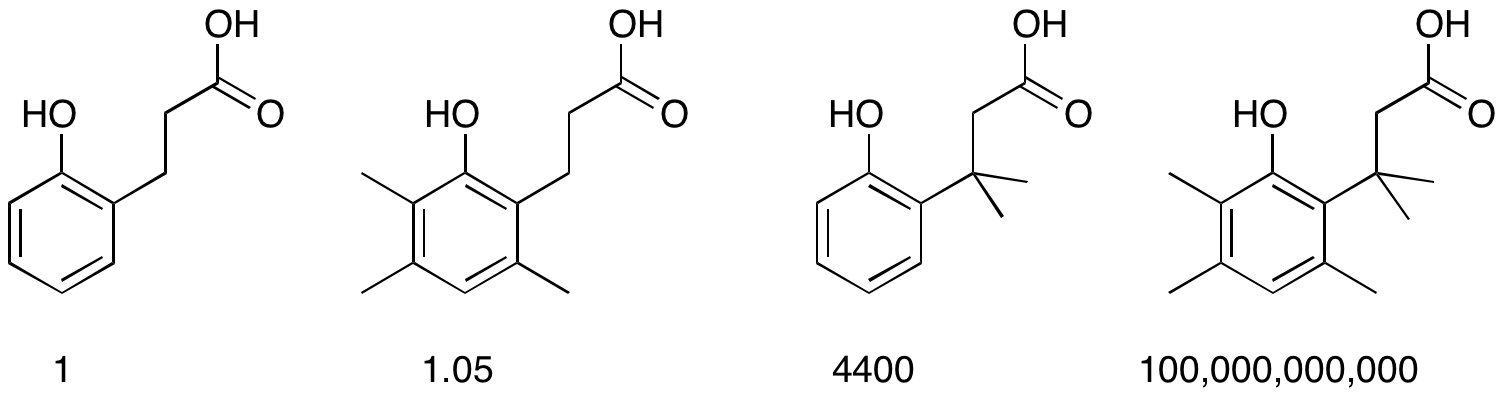
Thorpe–Ingold Effect
The Thorpe–Ingold effect, gem-dimethyl effect, or angle compression is an effect observed in chemistry where increasing steric hindrance favours ring closure and intramolecular reactions. The effect was first reported by Beesley, Thorpe, and Ingold in 1915 as part of a study of cyclization reactions. It has since been generalized to many areas of chemistry. The comparative rates of lactone formation (lactonization) of various 2-hydroxybenzenepropionic acids illustrate the effect. The placement of an increasing number of methyl groups accelerates the cyclization process. : One application of this effect is addition of a quaternary carbon (e.g., a gem-dimethyl group) in an alkyl chain to increase the reaction rate and/or equilibrium constant of cyclization reactions. An example of this is an olefin metathesis reaction: In the field of peptide foldamers, amino acid residues containing quaternary carbons such as 2-aminoisobutyric acid are used to promote formation of certain type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl
In organic chemistry, propyl is a three-carbon alkyl substituent with chemical formula for the linear form. This substituent form is obtained by removing one hydrogen atom attached to the terminal carbon of propane. A propyl substituent is often represented in organic chemistry with the symbol Pr (not to be confused with the element praseodymium). An isomeric form of propyl is obtained by moving the point of attachment from a terminal carbon atom to the central carbon atom, named 1-methylethyl or isopropyl. To maintain four substituents on each carbon atom, one hydrogen atom has to be moved from the middle carbon atom to the carbon atom which served as attachment point in the ''n''-propyl variant, written as . Linear propyl is sometimes termed normal and hence written with a prefix ''n''- (i.e., ''n-''propyl), as the absence of the prefix ''n''- does not indicate which attachment point is chosen, i.e. absence of prefix does not automatically exclude the possibility of it being ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beilstein Journal Of Organic Chemistry
The ''Beilstein Journal of Organic Chemistry'' is a peer-reviewed open-access scientific journal established in 2005. It is published by the Beilstein Institute for the Advancement of Chemical Sciences, a German non-profit foundation. The editor-in-chief is Peter Seeberger (Max Planck Institute of Colloids and Interfaces). According to the ''Journal Citation Reports'', the journal has a 2020 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 2.88. Scientific videos are available for selected articles of the journal. References External links * Organic chemistry journals Open access journals Creative Commons Attribution-licensed journals Publications established in 2005 English-language journals BioMed Central academic journals {{chem-journal-stu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pauson–Khand Reaction
The Pauson–Khand reaction (or PKR or PK-type reaction) is a chemical reaction described as a 2+2+1.html" ;"title="/nowiki>2+2+1">/nowiki>2+2+1/nowiki> cycloaddition between an alkyne, an alkene and carbon monoxide to form a α,β-cyclopentenone. Ihsan Ullah Khand (1935-1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde in Glasgow. Pauson and Khand's initial findings were intermolecular in nature, but starting a decade after the reaction's discovery, many intramolecular examples have been highlighted in both synthesis and methodology reports. This reaction was originally mediated by stoichiometric amounts of dicobalt octacarbonyl, but newer versions are both more efficient, enhancing reactivity and yield via utilizing different chiral auxiliaries for stereo induction, main group transition-metals (Ti, Mo, W, Fe, Co, Ni, Ru, Rh, Ir and Pd), and additives. Mechanism Whil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silyl Acetal
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1846 Von Ebelman's had synthesized Tetraethyl orthosilicate (Si(OC2H5)4). In 1863 Friedel and Crafts managed to make the first organosilieon compound with C-Si bonds which gone byound the syntheses of orthosilicic acid esters. The same year they also described a «polysilicic acid ether» in the preparation of ethyl- and methyl-o-silicic acid. The early extensive research in the field of organosilicon compounds was pioneerd in the beginning of 20th century by Frederic Kipping. He also had coined the term «silicone» (akin to ketones) in relation to these materials in 1904. In recognition of Kipping's achie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silyl Ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting groups for alcohols in organic synthesis. Since R1R2R3 can be combinations of differing groups which can be varied in order to provide a number of silyl ethers, this group of chemical compounds provides a wide spectrum of selectivity for protecting group chemistry. Common silyl ethers are: trimethylsilyl (TMS), ''tert''-butyldiphenylsilyl (TBDPS), ''tert''-butyldimethylsilyl (TBS/TBDMS) and triisopropylsilyl (TIPS). They are particularly useful because they can be installed and removed very selectively under mild conditions. Common silyl ethers Formation Commonly silylation of alcohols requires a silyl chloride and an amine base. One reliable and rapid procedure is the Corey protocol in which the alcohol is reacted with a silyl chlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boronic Ester
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic acids, etc. (molecules with vicinal, (1,2) or occasionally (1,3) substituted Lewis base donors (alcohol, amine, carboxylate)). The p''K''a of a boronic acid is ~9, but they can form tetrahedral boronate complexes with p''K''a ~7. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes. Boronic acids are used extensively in organic chemistry as chemical building blocks and intermediates predominantly in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Ester
In organic chemistry, a carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is and they are related to esters (), ethers () and also to the inorganic carbonates. Monomers of polycarbonate (e.g. Makrolon or Lexan) are linked by carbonate groups. These polycarbonates are used in eyeglass lenses, compact discs, and bulletproof glass. Small carbonate esters like dimethyl carbonate, ethylene carbonate, propylene carbonate are used as solvents, dimethyl carbonate is also a mild methylating agent. Structures Carbonate esters have planar OC(OC)2 cores, which confers rigidity. The unique O=C bond is short (1.173 Å in the depicted example), while the C-O bonds are more ether-like (the bond distances of 1.326 Å for the example depicted). Carbonate esters can be divided into three structural classes: acyclic, cyclic, and polymeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tether
A tether is a cord, fixture, or flexible attachment that characteristically anchors something movable to something fixed; it also maybe used to connect two movable objects, such as an item being towed by its tow. Applications for tethers include: fall arrest systems, lanyards, balloons, kites, airborne wind-power systems, anchors, floating water power systems, towing, animal constraint, space walks, power kiteing, and anti-theft devices. Failure Failure modes for tethers are considered in their design. A cord or rope tether may reach its breaking strength and fail. Outcomes can include an injury or fatal fall, and damage or loss of life to personnel or bystanders caused by backlash of the ruptured segments. Failure-prevention may be designed into a tethering system. Some safety harnesses are used in combination with a shock-absorbing lanyard, which has break-away stitching designed into it to prevent material failure and regulate deceleration, thereby preventing a serious ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |