Pauson–Khand Reaction on:
[Wikipedia]
[Google]
[Amazon]
 The Pauson–Khand (PK) reaction is a
The Pauson–Khand (PK) reaction is a /nowiki>2+2+1 /nowiki>

 But the alkene itself struggles to discriminate between the C4 and C5 position, unless the C2 position is sterically congested or the alkene has a chelating heteroatom.
The reaction's poor selectivity is ameliorated in
But the alkene itself struggles to discriminate between the C4 and C5 position, unless the C2 position is sterically congested or the alkene has a chelating heteroatom.
The reaction's poor selectivity is ameliorated in  Generally, the reaction is highly ''syn''-selective about the bridgehead hydrogen and substituents on the cyclopentane.
Generally, the reaction is highly ''syn''-selective about the bridgehead hydrogen and substituents on the cyclopentane.
 Appropriate chiral ligands or auxiliaries can make the reaction enantioselective (see ).
Appropriate chiral ligands or auxiliaries can make the reaction enantioselective (see ).
 Typical Pauson-Khand conditions are elevated temperatures and pressures in aromatic hydrocarbon (benzene, toluene) or ethereal (
Typical Pauson-Khand conditions are elevated temperatures and pressures in aromatic hydrocarbon (benzene, toluene) or ethereal (
 Lewis basic additives, such as ''n''-BuSMe, are also believed to accelerate the decarbonylative ligand exchange process. However, an alternative view holds that the additives make olefin insertion irreversible instead. Sulfur compounds are typically hard to handle and smelly, but n-dodecyl methyl sulfide and tetramethylthiourea do not suffer from those problems and can improve reaction performance.
Lewis basic additives, such as ''n''-BuSMe, are also believed to accelerate the decarbonylative ligand exchange process. However, an alternative view holds that the additives make olefin insertion irreversible instead. Sulfur compounds are typically hard to handle and smelly, but n-dodecyl methyl sulfide and tetramethylthiourea do not suffer from those problems and can improve reaction performance.
 ''N''-oxide additives can also improve enantio- and diastereoselectivity, although the mechanism thereby is not clear.
''N''-oxide additives can also improve enantio- and diastereoselectivity, although the mechanism thereby is not clear.

 One stabilization method is to generate the catalyst ''in situ''. Chung reports that Co(acac)2 can serve as a precatalyst, activated by
One stabilization method is to generate the catalyst ''in situ''. Chung reports that Co(acac)2 can serve as a precatalyst, activated by

 Heteroatoms are also acceptable: Mukai ''et al''
Heteroatoms are also acceptable: Mukai ''et al''
/nowiki>2+2+1cycloaddition, although this reactant is too active to store in bulk. Instead,  An example of a newer version is the use of the chlorodicarbonylrhodium(I) dimer, CO)2RhClsub>2, in the synthesis of (+)-phorbol by
An example of a newer version is the use of the chlorodicarbonylrhodium(I) dimer, CO)2RhClsub>2, in the synthesis of (+)-phorbol by
 The Pauson–Khand (PK) reaction is a
The Pauson–Khand (PK) reaction is a chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
, described as a cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
. In it, an alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
, an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
, and carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
combine into a α,β-cyclopentenone
2-Cyclopentenone is the organic compound with the chemical formula . 2-Cyclopentenone contains two functional groups, a ketone and an alkene. It is a colorless liquid. Its isomer, 3-cyclopentenone is less commonly encountered.
The term cyclopent ...
in the presence of a metal-carbonyl catalyst
Ihsan Ullah Khand
''Ihsan'' ( , also romanized ''ehsan'') is an Arabic term meaning "to do beautiful things", "beautification", "perfection", or "excellence" (Arabic: , ). ''Ihsan'' is a matter of taking one's inner faith ('' iman'') and showing it in both deed ...
(1935–1980) discovered the reaction around 1970, while working as a postdoctoral associate with Peter Ludwig Pauson (1925–2013) at the University of Strathclyde
The University of Strathclyde () is a public research university located in Glasgow, Scotland. Founded in 1796 as the Andersonian Institute, it is Glasgow's second-oldest university, having received its royal charter in 1964 as the first techn ...
in Glasgow. Pauson and Khand's initial findings were intermolecular in nature, but the reaction has poor selectivity. Some modern applications instead apply the reaction for intramolecular ends.
The traditional reaction requires a stoichiometric amounts of dicobalt octacarbonyl
Dicobalt octacarbonyl is an organocobalt compound with composition . This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent ...
, stabilized by a carbon monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the si ...
atmosphere
An atmosphere () is a layer of gases that envelop an astronomical object, held in place by the gravity of the object. A planet retains an atmosphere when the gravity is great and the temperature of the atmosphere is low. A stellar atmosph ...
. Catalytic metal quantities, enhanced reactivity and yield, or stereoinduction are all possible with the right chiral auxiliaries
In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the st ...
, choice of transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
(Ti, Mo, W, Fe, Co, Ni, Ru, Rh, Ir and Pd), and additives.
Mechanism
While the mechanism has not yet been fully elucidated, Magnus' 1985 explanation is widely accepted for both mono- and dinuclear catalysts, and was corroborated by computational studies published by Nakamura and Yamanaka in 2001. The reaction starts withdicobalt hexacarbonyl acetylene complex
Dicobalt hexacarbonyl acetylene complexes are a family of In organocobalt compounds with the formula . A large variety of R groups are tolerated. They are red compounds that are soluble in organic solvents. They arise from the reaction of alkynes ...
. Binding of an alkene gives a metallacyclopentene complex. CO then migratorily inserts into an M-C bond. Reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
delivers the cyclopentenone
2-Cyclopentenone is the organic compound with the chemical formula . 2-Cyclopentenone contains two functional groups, a ketone and an alkene. It is a colorless liquid. Its isomer, 3-cyclopentenone is less commonly encountered.
The term cyclopent ...
. Typically, the dissociation of carbon monoxide from the organometallic complex is rate limiting.

Selectivity
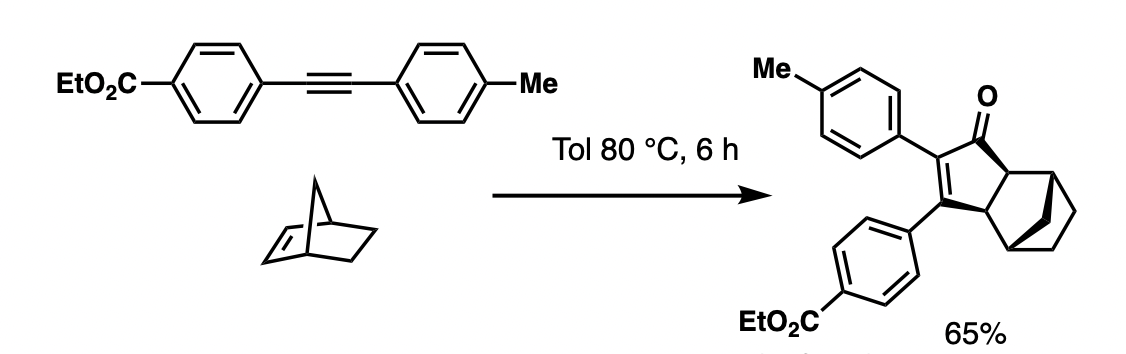
The reaction works with both terminal and internal alkynes, although internal alkynes tend to give lower yields. The order of reactivity for the alkene is(strained cyclic) > (terminal) > (disubstituted) > (trisubstituted).Tetrasubstituted alkenes and alkenes with strongly electron-withdrawing groups are unsuitable. With unsymmetrical alkenes or alkynes, the reaction is rarely
regioselective
In organic chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a str ...
, although some patterns can be observed.
For mono-substituted alkenes, alkyne substituents typically direct: larger groups prefer the C2 position, and electron-withdrawing groups prefer the C3 position.
 But the alkene itself struggles to discriminate between the C4 and C5 position, unless the C2 position is sterically congested or the alkene has a chelating heteroatom.
The reaction's poor selectivity is ameliorated in
But the alkene itself struggles to discriminate between the C4 and C5 position, unless the C2 position is sterically congested or the alkene has a chelating heteroatom.
The reaction's poor selectivity is ameliorated in intramolecular reaction
In chemistry, intramolecular describes a Chemical process, process or characteristic limited within the Chemical structure, structure of a single molecule, a property or phenomenon limited to the extent of a single molecule.
Relative rates
In i ...
s. For this reason, the intramolecular Pauson-Khand is common in total synthesis, particularly the formation of 5,5- and 6,5-membered fused bicycles
A bicycle, also called a pedal cycle, bike, push-bike or cycle, is a human-powered or motor-assisted, pedal-driven, single-track vehicle, with two wheels attached to a frame, one behind the other. A is called a cyclist, or bicyclist.
...
.
 Generally, the reaction is highly ''syn''-selective about the bridgehead hydrogen and substituents on the cyclopentane.
Generally, the reaction is highly ''syn''-selective about the bridgehead hydrogen and substituents on the cyclopentane.
 Appropriate chiral ligands or auxiliaries can make the reaction enantioselective (see ).
Appropriate chiral ligands or auxiliaries can make the reaction enantioselective (see ). BINAP
BINAP (2,2′-bis(diphenylphosphino)-1,1′-binaphthyl) is an organophosphorus compound. This Optical isomerism, chiral diphosphines, diphosphine ligand is widely used in chiral synthesis, asymmetric synthesis. It consists of a pair of 2-diphe ...
is commonly employed.
Additives
 Typical Pauson-Khand conditions are elevated temperatures and pressures in aromatic hydrocarbon (benzene, toluene) or ethereal (
Typical Pauson-Khand conditions are elevated temperatures and pressures in aromatic hydrocarbon (benzene, toluene) or ethereal (tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
, 1,2-dichloroethane) solvents. These harsh conditions may be attenuated with the addition of various additives.

Absorbent surfaces
Adsorbing the metallic complex onto silica or alumina can enhance the rate of decarbonylative ligand exchange as exhibited in the image below. This is because the donor posits itself on a solid surface (i.e. silica). Additionally using a solid support restricts conformational movement ( rotamer effect).Lewis bases
Traditional catalytic aids such as phosphine ligands make the cobalt complex too stable, but bulky phosphite ligands are operable. Lewis basic additives, such as ''n''-BuSMe, are also believed to accelerate the decarbonylative ligand exchange process. However, an alternative view holds that the additives make olefin insertion irreversible instead. Sulfur compounds are typically hard to handle and smelly, but n-dodecyl methyl sulfide and tetramethylthiourea do not suffer from those problems and can improve reaction performance.
Lewis basic additives, such as ''n''-BuSMe, are also believed to accelerate the decarbonylative ligand exchange process. However, an alternative view holds that the additives make olefin insertion irreversible instead. Sulfur compounds are typically hard to handle and smelly, but n-dodecyl methyl sulfide and tetramethylthiourea do not suffer from those problems and can improve reaction performance.
Amine ''N''-oxides
The two most common amine ''N''-oxides are ''N''-methylmorpholine ''N''-oxide (NMO) and trimethylamine ''N''-oxide (TMANO). It is believed that these additives remove carbon monoxide ligands via nucleophilic attack of the ''N''-oxide onto the CO carbonyl, oxidizing the CO into CO2, and generating an unsaturated organometallic complex. This renders the first step of the mechanism irreversible, and allows for more mild conditions.Hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understo ...
s of the aforementioned amine ''N''-oxides have similar effect.
 ''N''-oxide additives can also improve enantio- and diastereoselectivity, although the mechanism thereby is not clear.
''N''-oxide additives can also improve enantio- and diastereoselectivity, although the mechanism thereby is not clear.

Alternative catalysts
(Co)4(CO)12 and Co3(CO)9(μ3-CH) also catalyze the PK reaction although Takayama ''et al'' detail a reaction catalyzed bydicobalt octacarbonyl
Dicobalt octacarbonyl is an organocobalt compound with composition . This metal carbonyl is used as a reagent and catalyst in organometallic chemistry and organic synthesis, and is central to much known organocobalt chemistry. It is the parent ...
.
 One stabilization method is to generate the catalyst ''in situ''. Chung reports that Co(acac)2 can serve as a precatalyst, activated by
One stabilization method is to generate the catalyst ''in situ''. Chung reports that Co(acac)2 can serve as a precatalyst, activated by sodium borohydride
Sodium borohydride, also known as sodium tetrahydridoborate and sodium tetrahydroborate, is an inorganic compound with the formula (sometimes written as ). It is a white crystalline solid, usually encountered as an aqueous basic solution. Sodi ...
.
Other metals
catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
requires a silver triflate
Silver trifluoromethanesulfonate, or silver triflate is the triflate (CF3SO3−) salt of Ag+. It is a white or colorless solid that is soluble in water and some organic solvents including, benzene. It is a reagent used in the synthesis of organi ...
co-catalyst to effect the Pauson–Khand reaction:

Molybdenum hexacarbonyl
Molybdenum hexacarbonyl (also called molybdenum carbonyl) is the chemical compound with the formula Mo(CO)6. This colorless solid, like its chromium, tungsten, and seaborgium analogues, is noteworthy as a volatile, air-stable derivative of a metal ...
is a carbon monoxide donor in PK-type reactions between allene
In organic chemistry, allenes are organic compounds in which one carbon atom has double bonds with each of its two adjacent carbon atoms (, where R is hydrogen, H or some organyl group). Allenes are classified as diene#Classes, cumulated dienes ...
s and alkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s with dimethyl sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is ...
in toluene. Titanium, nickel, and zirconium complexes admit the reaction. Other metals can also be employed in these transformations.
Substrate tolerance
In general allenes, support the Pauson–Khand reaction; regioselectivity is determined by the choice of metal catalyst. Density functional investigations show the variation arises from different transition state metal geometries. Heteroatoms are also acceptable: Mukai ''et al''
Heteroatoms are also acceptable: Mukai ''et al''carbodiimide
In organic chemistry, a carbodiimide (systematic IUPAC name: methanediimine) is a functional group with the formula RN=C=NR. On Earth they are exclusively synthetic, but in interstellar space the parent compound HN=C=NH has been detected by its ...
.

Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is ...
also lends itself to a ceric ammonium nitrate
Ceric ammonium nitrate (CAN) is the inorganic compound with the formula . This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard oxidant in quantitative analysis.
Preparation, properties, ...
cyclobutadiene is generated in situ from decomplexation
In chemistry, decomplexation refers to the removal of a ligand from a coordination complex. Decomplexation is of particular interest when the ligand has been synthesized within the coordination sphere of the metal, as is often the case in organome ...
of stable cyclobutadiene iron tricarbonyl with (CAN).
 An example of a newer version is the use of the chlorodicarbonylrhodium(I) dimer, CO)2RhClsub>2, in the synthesis of (+)-phorbol by
An example of a newer version is the use of the chlorodicarbonylrhodium(I) dimer, CO)2RhClsub>2, in the synthesis of (+)-phorbol by Phil Baran
Phil S. Baran (born August 10, 1977) is a synthetic organic chemist and Professor in the Department of Chemistry at the Scripps Research Institute.cyclopentenone
2-Cyclopentenone is the organic compound with the chemical formula . 2-Cyclopentenone contains two functional groups, a ketone and an alkene. It is a colorless liquid. Its isomer, 3-cyclopentenone is less commonly encountered.
The term cyclopent ...
as well as 7-membered ring.
Carbon monoxide generation ''in situ''
The cyclopentenone motif can be prepared from aldehydes, carboxylic acids, and formates. These examples typically employ rhodium as the catalyst, as it is commonly used indecarbonylation
In chemistry, decarbonylation is a type of organic reaction that involves the loss of carbon monoxide (CO). It is often an undesirable reaction, since it represents a degradation. In the chemistry of metal carbonyls, decarbonylation describes a ...
reactions. The decarbonylation and PK reaction occur in the same reaction vessel.
See also
*Nicholas reaction The Nicholas reaction is an organic reaction where a dicobalt octacarbonyl-stabilized propargylic cation is reacted with a nucleophile. Oxidative demetallation gives the desired alkylated alkyne. It is named after Kenneth M. Nicholas.
Sever ...
Further reading
For Khand and Pauson's perspective on the reaction: * * * For a modern perspective: * * * *References
{{Alkynes Cycloadditions Multiple component reactions Name reactions