|
Inorganic Carbon
Total inorganic carbon (''C''T or TIC) is the sum of the inorganic carbon species. Carbon Chemical compound, compounds can be distinguished as either Organic compound, organic or inorganic, and Dissolved organic carbon, dissolved or Particulate organic matter, particulate, depending on their composition. Organic carbon forms the backbone of key components of organic compounds such as proteins, lipids, carbohydrates, and nucleic acids. Inorganic carbon is found primarily in simple compounds such as carbon dioxide (), carbonic acid (), bicarbonate (), and carbonate (). Overview The aquatic inorganic carbon system is composed of the various ionic, dissolved, solid, and/or gaseous forms of carbon dioxide in water. These species include Carbon dioxide, dissolved carbon dioxide, carbonic acid, bicarbonate anion, Carbonate, carbonate anion, calcium carbonate, magnesium carbonate, and others. The relative amounts of each species in a body of water depends on physical variables includin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
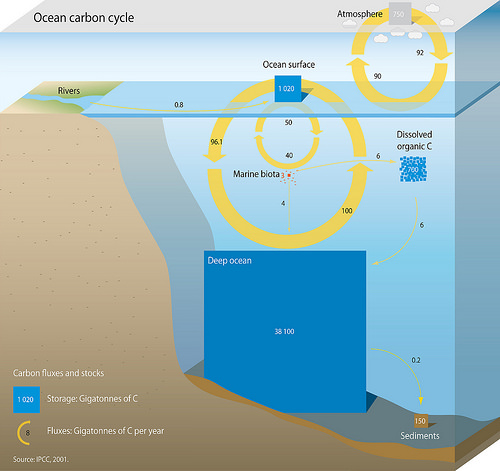
Marine Carbon Cycle
The oceanic carbon cycle (or marine carbon cycle) is composed of processes that exchange carbon between various pools within the ocean as well as between the atmosphere, Earth interior, and the seafloor. The carbon cycle is a result of many interacting forces across multiple time and space scales that circulates carbon around the planet, ensuring that carbon is available globally. The Oceanic carbon cycle is a central process to the global carbon cycle and contains both inorganic carbon (carbon not associated with a living thing, such as carbon dioxide) and organic carbon (carbon that is, or has been, incorporated into a living thing). Part of the marine carbon cycle transforms carbon between non-living and living matter. Three main processes (or pumps) that make up the marine carbon cycle bring atmospheric carbon dioxide (CO2) into the ocean interior and distribute it through the oceans. These three pumps are: (1) the solubility pump, (2) the carbonate pump, and (3) the biologi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limnology
Limnology ( ; from Greek λίμνη, ''limne'', "lake" and λόγος, ''logos'', "knowledge") is the study of inland aquatic ecosystems. The study of limnology includes aspects of the biological, chemical, physical, and geological characteristics of fresh and saline, natural and man-made bodies of water. This includes the study of lakes, reservoirs, ponds, rivers, springs, streams, wetlands, and groundwater.Wetzel, R.G. 2001. Limnology: Lake and River Ecosystems, 3rd ed. Academic Press () Water systems are often categorized as either running (lotic) or standing (lentic). Limnology includes the study of the drainage basin, movement of water through the basin and biogeochemical changes that occur en route. A more recent sub-discipline of limnology, termed landscape limnology, studies, manages, and seeks to conserve these ecosystems using a landscape perspective, by explicitly examining connections between an aquatic ecosystem and its drainage basin. Recently, the need to underst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oceanography
Oceanography (), also known as oceanology and ocean science, is the scientific study of the oceans. It is an Earth science, which covers a wide range of topics, including ecosystem dynamics; ocean currents, waves, and geophysical fluid dynamics; plate tectonics and the geology of the sea floor; and fluxes of various chemical substances and physical properties within the ocean and across its boundaries. These diverse topics reflect multiple disciplines that oceanographers utilize to glean further knowledge of the world ocean, including astronomy, biology, chemistry, climatology, geography, geology, hydrology, meteorology and physics. Paleoceanography studies the history of the oceans in the geologic past. An oceanographer is a person who studies many matters concerned with oceans, including marine geology, physics, chemistry and biology. History Early history Humans first acquired knowledge of the waves and currents of the seas and oceans in pre-historic times. Observations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PCO2
''p''CO2, pCO2, or P_\ceis the partial pressure of carbon dioxide (CO2), often used in reference to blood but also used in meteorology, climate science, oceanography, and limnology to describe the fractional pressure of CO2 as a function of its concentration in gas or dissolved phases. The units of ''p''CO2 are mmHg, atm, torr, Pa, or any other standard unit of atmospheric pressure. The ''p''CO2 of Earth's atmosphere has risen from approximately 280 ppm (parts-per-million) to a mean 2019 value of 409.8 ppm as a result of anthropogenic release of carbon dioxide from fossil fuel burning. This is the highest atmospheric concentration to have existed on Earth for at least the last 800,000 years. Medicine In medicine, the partial pressure of carbon dioxide in arterial blood is called P_ or PaCO2. Measurement of P_ in the systemic circulation indicates the effectiveness of ventilation at the lungs' alveoli, given the diffusing capacity of the gas. It is a good indicator of res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissolved Inorganic Carbon
Dissolved inorganic carbon (DIC) is the sum of the aqueous species of inorganic carbon in a solution. Carbon compounds can be distinguished as either organic or inorganic, and as dissolved or particulate, depending on their composition. Organic carbon forms the backbone of key component of organic compounds such as – proteins, lipids, carbohydrates, and nucleic acids. Inorganic carbon is found primarily in simple compounds such as carbon dioxide, carbonic acid, bicarbonate, and carbonate (CO2, H2CO3, , respectively). Dissolved inorganic carbon (DIC) includes three major aqueous species, CO2, ,, and to a lesser extent their complexes in solution with metal ions. Marine ecosystems Solubility pump Aqueous carbon dioxide reacts with water to form carbonic acid which is very unstable and will dissociate rapidly into hydronium and bicarbonate. Therefore, in seawater, dissolved inorganic carbon is commonly referred to as the collection of bicarbonate, carbonate ions, and dissolv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. It is measured by titrating the solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter of calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant. Although alkalinity is primarily a term used by oceanographers, it is also used by hydrologists to describe temporary hardness. Moreover, measuring alkalinity is important in determining a stream's ability to neutralize acidic pollution from rainfall or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Carbon
Total inorganic carbon (''C''T or TIC) is the sum of the inorganic carbon species. Carbon Chemical compound, compounds can be distinguished as either Organic compound, organic or inorganic, and Dissolved organic carbon, dissolved or Particulate organic matter, particulate, depending on their composition. Organic carbon forms the backbone of key components of organic compounds such as proteins, lipids, carbohydrates, and nucleic acids. Inorganic carbon is found primarily in simple compounds such as carbon dioxide (), carbonic acid (), bicarbonate (), and carbonate (). Overview The aquatic inorganic carbon system is composed of the various ionic, dissolved, solid, and/or gaseous forms of carbon dioxide in water. These species include Carbon dioxide, dissolved carbon dioxide, carbonic acid, bicarbonate anion, Carbonate, carbonate anion, calcium carbonate, magnesium carbonate, and others. The relative amounts of each species in a body of water depends on physical variables includin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food, energy or organic micronutrients. Its chemical formula, H2O, indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. "Water" is also the name of the liquid state of H2O at standard temperature and pressure. A number of natural states of water exist. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice may precipitate in the form of snow. The gaseous state of water is steam or water vapor. Water co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T are p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CO2SYS
CO2SYS is a family of software programs that calculate chemical equilibria for aquatic inorganic carbon species and parameters. Their core function is to use any two of the four central inorganic carbon system parameters ( pH, alkalinity, dissolved inorganic carbon, and partial pressure of carbon dioxide) to calculate various chemical properties of the system. These programs are widely used by oceanographers and limnologists to understand and predict chemical equilibria in natural waters. History Chemical equilibria in marine and freshwater systems were calculated according to various conventions for most of the 20th century, which led to discrepancies among laboratories' calculations and limited scientific reproducibility. CO2SYS was first published by Ernie Lewis and Doug Wallace in 1998 as a DOS-interface program written in QBasic. Subsequent developments have included several MATLAB implementations, two Microsoft Excel templates, a Python package "PyCO2SYS", and an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |