|
CO2SYS
CO2SYS is a family of software programs that calculate chemical equilibria for aquatic inorganic carbon species and parameters. Their core function is to use any two of the four central inorganic carbon system parameters ( pH, alkalinity, dissolved inorganic carbon, and partial pressure of carbon dioxide) to calculate various chemical properties of the system. These programs are widely used by oceanographers and limnologists to understand and predict chemical equilibria in natural waters. History Chemical equilibria in marine and freshwater systems were calculated according to various conventions for most of the 20th century, which led to discrepancies among laboratories' calculations and limited scientific reproducibility. CO2SYS was first published by Ernie Lewis and Doug Wallace in 1998 as a DOS-interface program written in QBasic. Subsequent developments have included several MATLAB implementations, two Microsoft Excel templates, a Python package "PyCO2SYS", and an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Carbon
Total inorganic carbon (''C''T or TIC) is the sum of the inorganic carbon species. Carbon Chemical compound, compounds can be distinguished as either Organic compound, organic or inorganic, and Dissolved organic carbon, dissolved or Particulate organic matter, particulate, depending on their composition. Organic carbon forms the backbone of key components of organic compounds such as proteins, lipids, carbohydrates, and nucleic acids. Inorganic carbon is found primarily in simple compounds such as carbon dioxide (), carbonic acid (), bicarbonate (), and carbonate (). Overview The aquatic inorganic carbon system is composed of the various ionic, dissolved, solid, and/or gaseous forms of carbon dioxide in water. These species include Carbon dioxide, dissolved carbon dioxide, carbonic acid, bicarbonate anion, Carbonate, carbonate anion, calcium carbonate, magnesium carbonate, and others. The relative amounts of each species in a body of water depends on physical variables includin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkalinity
Alkalinity (from ar, القلوي, al-qaly, lit=ashes of the saltwort) is the capacity of water to resist acidification. It should not be confused with basicity, which is an absolute measurement on the pH scale. Alkalinity is the strength of a buffer solution composed of weak acids and their conjugate bases. It is measured by titrating the solution with an acid such as HCl until its pH changes abruptly, or it reaches a known endpoint where that happens. Alkalinity is expressed in units of concentration, such as meq/L (milliequivalents per liter), μeq/kg (microequivalents per kilogram), or mg/L CaCO3 (milligrams per liter of calcium carbonate). Each of these measurements corresponds to an amount of acid added as a titrant. Although alkalinity is primarily a term used by oceanographers, it is also used by hydrologists to describe temporary hardness. Moreover, measuring alkalinity is important in determining a stream's ability to neutralize acidic pollution from rainfall or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Software
Software is a set of computer programs and associated documentation and data. This is in contrast to hardware, from which the system is built and which actually performs the work. At the lowest programming level, executable code consists of machine language instructions supported by an individual processor—typically a central processing unit (CPU) or a graphics processing unit (GPU). Machine language consists of groups of binary values signifying processor instructions that change the state of the computer from its preceding state. For example, an instruction may change the value stored in a particular storage location in the computer—an effect that is not directly observable to the user. An instruction may also invoke one of many input or output operations, for example displaying some text on a computer screen; causing state changes which should be visible to the user. The processor executes the instructions in the order they are provided, unless it is instructed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anoxic Waters
Anoxic waters are areas of sea water, fresh water, or groundwater that are depleted of dissolved oxygen. The US Geological Survey defines anoxic groundwater as those with dissolved oxygen concentration of less than 0.5 milligrams per litre. Anoxic waters can be contrasted with hypoxic waters, which are low (but not lacking) in dissolved oxygen. This condition is generally found in areas that have restricted water exchange. In most cases, oxygen is prevented from reaching the deeper levels by a physical barrier, as well as by a pronounced density stratification, in which, for instance, heavier hypersaline waters rest at the bottom of a basin. Anoxic conditions will occur if the rate of oxidation of organic matter by bacteria is greater than the supply of dissolved oxygen. Anoxic waters are a natural phenomenon, and have occurred throughout geological history. The Permian–Triassic extinction event, a mass extinction of species from the world's oceans, may have resulted from w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Carbonate
Magnesium carbonate, (archaic name magnesia alba), is an inorganic salt that is a colourless or white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals. Forms The most common magnesium carbonate forms are the anhydrous salt called magnesite (), and the di, tri, and pentahydrates known as barringtonite (), nesquehonite (), and lansfordite (), respectively. Some basic forms such as artinite (), hydromagnesite (), and dypingite () also occur as minerals. All of those minerals are colouress or white. Magnesite consists of colourless or white trigonal crystals. The anhydrous salt is practically insoluble in water, acetone, and ammonia. All forms of magnesium carbonate react with acids. Magnesite crystallizes in the calcite structure wherein is surrounded by six oxygen atoms. The dihydrate has a triclinic structure, while the trihydrate has a monoclinic structure. References to "light" and "heavy" magnesium carbonates actually refer to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
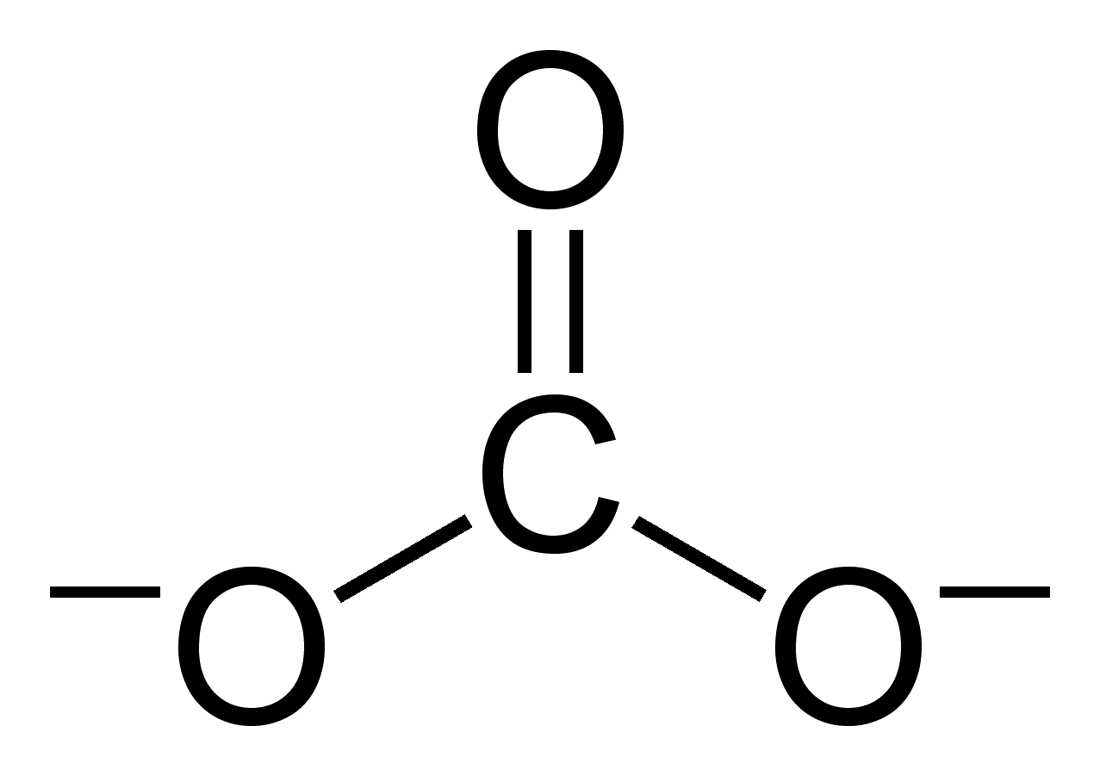
Carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid . The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid ; and the conjugate acid of , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide (chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Python (programming Language)
Python is a high-level, general-purpose programming language. Its design philosophy emphasizes code readability with the use of significant indentation. Python is dynamically-typed and garbage-collected. It supports multiple programming paradigms, including structured (particularly procedural), object-oriented and functional programming. It is often described as a "batteries included" language due to its comprehensive standard library. Guido van Rossum began working on Python in the late 1980s as a successor to the ABC programming language and first released it in 1991 as Python 0.9.0. Python 2.0 was released in 2000 and introduced new features such as list comprehensions, cycle-detecting garbage collection, reference counting, and Unicode support. Python 3.0, released in 2008, was a major revision that is not completely backward-compatible with earlier versions. Python 2 was discontinued with version 2.7.18 in 2020. Python consistently ranks as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |