|
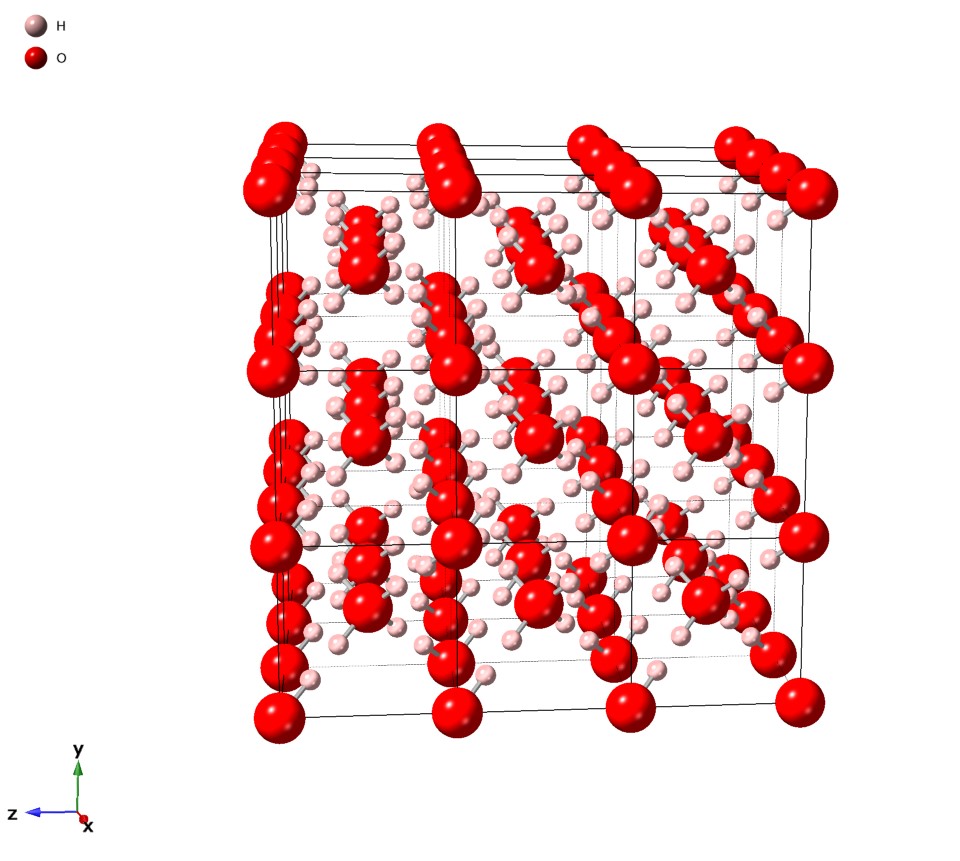
Ice VIII
Ice VIII is a tetragonal crystalline form of ice formed from ice VII by cooling it below 5 °C. It is more ordered than ice VII, since the hydrogen atoms assume fixed positions. Ordinary water ice is known as ice Ih, (in the Bridgman nomenclature). Different types of ice, from ice II to ice XVIII, have been created in the laboratory at different temperatures and pressures. Image:Iceviiistructure-ru.gif, The crystal structure of ice VIII See also *Ice Ice is water frozen into a solid state, typically forming at or below temperatures of 0 degrees Celsius or Depending on the presence of impurities such as particles of soil or bubbles of air, it can appear transparent or a more or less opaq ..., for other crystalline forms of ice References * {{inorganic-compound-stub Water ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice VII
Ice VII is a cubic crystalline form of ice. It can be formed from liquid water above 3 GPa (30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI below 95 K. (Different types of ice, from ice II to ice XVIII, have been created in the laboratory at different temperatures and pressures. Ordinary water ice is known as ice Ih in the Bridgman nomenclature.) Ice VII is metastable over a wide range of temperatures and pressures and transforms into low-density amorphous ice (LDA) above . Ice VII has a triple point with liquid water and ice VI at 355 K and 2.216 GPa, with the melt line extending to at least and 10 GPa. Ice VII can be formed within nanoseconds by rapid compression via shock-waves. It can also be created by increasing the pressure on ice VI at ambient temperature. At around 5 GPa, Ice VII becomes the tetragonal Ice VIIt. Like the majority of ice phases (including ice Ih), the hydrogen atom positions are dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice Ih
Photograph showing details of an ice cube under magnification. Ice Ih is the form of ice commonly seen on Earth. Phase space of ice Ih with respect to other ice phases. Ice Ih (hexagonal ice crystal) (pronounced: ice one h, also known as ice-phase-one) is the hexagonal crystal form of ordinary ice, or frozen water. Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic that is occasionally present in the upper atmosphere. Ice Ih exhibits many peculiar properties that are relevant to the existence of life and regulation of global climate. For a description of these properties, see ''Ice'', which deals primarily with ice Ih. The crystal structure is characterized by the oxygen atoms forming hexagonal symmetry with near tetrahedral bonding angles. Ice Ih is stable down to , as evidenced by x-ray diffraction and extremely high resolution thermal expansion measurements. Ice Ih is also stable under applied pressures of up to about where i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Percy Williams Bridgman
Percy Williams Bridgman (April 21, 1882 – August 20, 1961) was an American physicist who received the 1946 Nobel Prize in Physics for his work on the physics of high pressures. He also wrote extensively on the scientific method and on other aspects of the philosophy of science. The Bridgman effect, the Bridgman–Stockbarger technique, and the high-pressure mineral bridgmanite are named after him. Biography Early life Bridgman was born in Cambridge, Massachusetts, and grew up in nearby Auburndale. Bridgman's parents were both born in New England. His father, Raymond Landon Bridgman, was "profoundly religious and idealistic" and worked as a newspaper reporter assigned to state politics. His mother, Mary Ann Maria Williams, was described as "more conventional, sprightly, and competitive". Bridgman attended both elementary and high school in Auburndale, where he excelled at competitions in the classroom, on the playground, and while playing chess. Described as both shy and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice II
Ice II is a rhombohedral crystalline form of ice with a highly ordered structure. It is formed from ice Ih by compressing it at a temperature of 198 K at 300 MPa or by decompressing ice V. When heated it undergoes transformation to ice III. Ordinary water ice is known as ice Ih, (in the Bridgman nomenclature). Different types of ice, from ice II to ice XIX, have been created in the laboratory at different temperatures and pressures. It is thought that the cores of icy moons like Jupiter's Ganymede may be made of ice II. History The properties of ice II were first described and recorded by Gustav Heinrich Johann Apollon Tammann in 1900 during his experiments with ice under high pressure and low temperatures. Having produced ice III, Tammann then tried condensing the ice at a temperature between under of pressure. Tammann noted that in this state ice II was denser than he had observed ice III to be. He also found that both types of ice can be kept at normal atmospheric pres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superionic Water
Superionic water, also called superionic ice or ice XVIII is a phase of water that exists at extremely high temperatures and pressures. In superionic water, water molecules break apart and the oxygen ions crystallize into an evenly spaced lattice while the hydrogen ions float around freely within the oxygen lattice.Weird water lurking inside giant planets New Scientist,01 September 2010, Magazine issue 2776. The freely mobile hydrogen ions make superionic water almost as as typical metals, making it a [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |