|
Ice VI
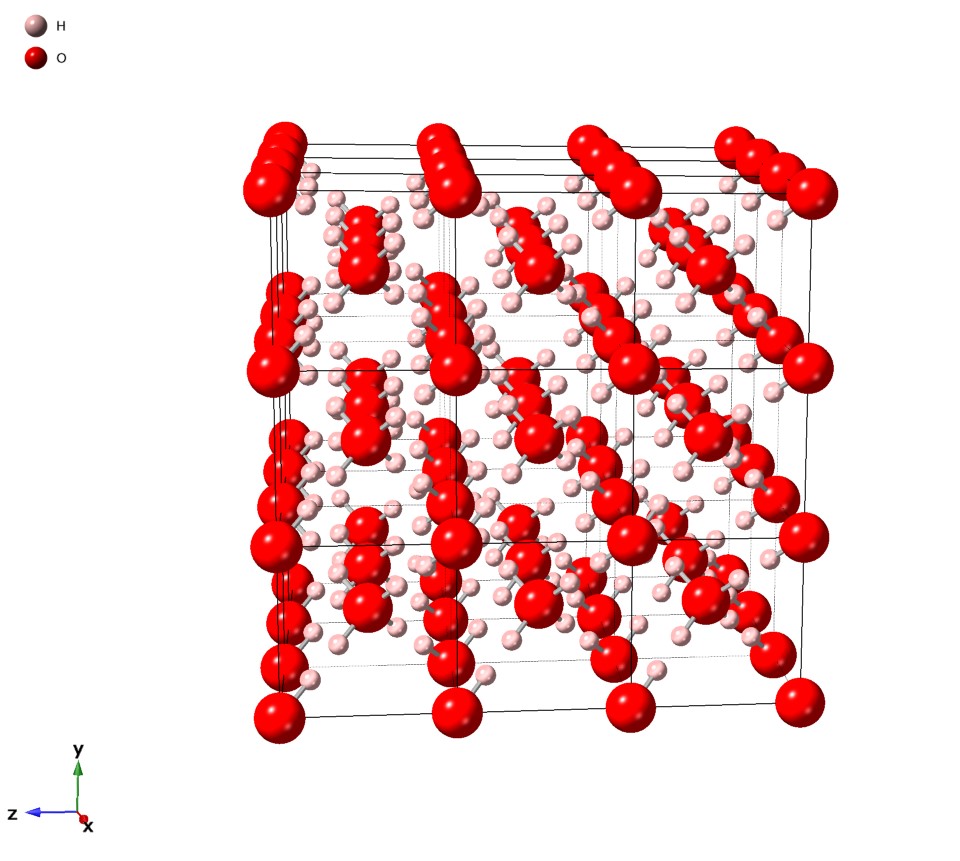
Ice VI is a form of ice that exists at high pressure at the order of about 1 GPa (= 10 000 bar) and temperatures ranging from 130 up to 355 Kelvin (−143 °C up to 82 °C); see also the phase diagram of water. Its discovery and the discovery of other high-pressure forms of water were published by P.W. Bridgman in January 1912. It is part of one of the inner layers of Titan. Properties Ice VI has a density of 1.31 g/cm3 and a tetragonal crystal system with the space group P42/nmc; its unit cell contains 10 water molecules and has the dimensions a=6.27 Å and c=5.79 Å. The triple point of ''ice VI'' with ice VII and liquid water is at about 82 °C and 2.22 GPa and its triple point with ice V and liquid water is at 0.16 °C and 0.6324 GPa = 6324 bar. www1.lsbu.ac.uk, version of 9 September 20 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagram Of Water
Phase or phases may refer to: Science *State of matter, or phase, one of the distinct forms in which matter can exist *Phase (matter), a region of space throughout which all physical properties are essentially uniform * Phase space, a mathematical space in which each possible state of a physical system is represented by a point — this equilibrium point is also referred to as a "microscopic state" **Phase space formulation, a formulation of quantum mechanics in phase space *Phase (waves), the position of a point in time (an instant) on a waveform cycle **Instantaneous phase, generalization for both cyclic and non-cyclic phenomena * AC phase, the phase offset between alternating current electric power in multiple conducting wires **Single-phase electric power, distribution of AC electric power in a system where the voltages of the supply vary in unison **Three-phase electric power, a common method of AC electric power generation, transmission, and distribution *Phase problem, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High Pressure
In science and engineering the study of high pressure examines its effects on materials and the design and construction of devices, such as a diamond anvil cell, which can create high pressure. By ''high pressure'' is usually meant pressures of thousands (kilobars) or millions (megabars) of times atmospheric pressure (about 1 bar or 100,000 Pa). History and overview Percy Williams Bridgman received a Nobel Prize in 1946 for advancing this area of physics by several magnitudes of pressure (400 MPa to 40,000 MPa). The list of founding fathers of this field includes also the names of Harry George Drickamer, Tracy Hall, Francis P. Bundy, Leonid F. Vereschagin, and Sergey M. Stishov. It was by applying high pressure as well as high temperature to carbon that man-made diamonds were first produced as well as many other interesting discoveries. Almost any material when subjected to high pressure will compact itself into a denser form, for example, quartz, also called silica or silicon di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bar (unit)
The bar is a metric unit of pressure, but not part of the International System of Units (SI). It is defined as exactly equal to 100,000 Pa (100 kPa), or slightly less than the current average atmospheric pressure on Earth at sea level (approximately 1.013 bar). By the barometric formula, 1 bar is roughly the atmospheric pressure on Earth at an altitude of 111 metres at 15 °C. The bar and the millibar were introduced by the Norwegian meteorologist Vilhelm Bjerknes, who was a founder of the modern practice of weather forecasting. The International System of Units, despite previously mentioning the bar, now omits any mention of it.. The bar has been legally recognised in countries of the European Union since 2004.British Standard BS 350:2004 ''Conversion Factors for Units''. The US National Institute of Standards and Technology (NIST) deprecates its use except for "limited use in meteorology" and lists it as one of several units that "must not be introduced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Percy Williams Bridgman
Percy Williams Bridgman (April 21, 1882 – August 20, 1961) was an American physicist who received the 1946 Nobel Prize in Physics for his work on the physics of high pressures. He also wrote extensively on the scientific method and on other aspects of the philosophy of science. The Bridgman effect, the Bridgman–Stockbarger technique, and the high-pressure mineral bridgmanite are named after him. Biography Early life Bridgman was born in Cambridge, Massachusetts, and grew up in nearby Auburndale. Bridgman's parents were both born in New England. His father, Raymond Landon Bridgman, was "profoundly religious and idealistic" and worked as a newspaper reporter assigned to state politics. His mother, Mary Ann Maria Williams, was described as "more conventional, sprightly, and competitive". Bridgman attended both elementary and high school in Auburndale, where he excelled at competitions in the classroom, on the playground, and while playing chess. Described as both shy and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titan (moon)
Titan is the largest moon of Saturn and the second-largest natural satellite in the Solar System. It is the only moon known to have a dense atmosphere, and is the only known object in space other than Earth on which clear evidence of stable bodies of surface liquid has been found. Titan is one of the seven gravitationally rounded moons in orbit around Saturn, and the second most distant from Saturn of those seven. Frequently described as a planet-like moon, Titan is 50% larger (in diameter) than Earth's Moon and 80% more massive. It is the second-largest moon in the Solar System after Jupiter's moon Ganymede, and is larger than the planet Mercury, but only 40% as massive. Discovered in 1655 by the Dutch astronomer Christiaan Huygens, Titan was the first known moon of Saturn, and the sixth known planetary satellite (after Earth's moon and the four Galilean moons of Jupiter). Titan orbits Saturn at 20 Saturn radii. From Titan's surface, Saturn subtends an arc of 5.09 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal Crystal System
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., pp. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Space Groups
There are 230 space groups in three dimensions, given by a number index, and a full name in Hermann–Mauguin notation, and a short name (international short symbol). The long names are given with spaces for readability. The groups each have a point group of the unit cell. Symbols In Hermann–Mauguin notation, space groups are named by a symbol combining the point group identifier with the uppercase letters describing the lattice type. Translations within the lattice in the form of screw axes and glide planes are also noted, giving a complete crystallographic space group. These are the Bravais lattices in three dimensions: *P primitive *I body centered (from the German ''Innenzentriert'') *F face centered (from the German ''Flächenzentriert'') *A centered on A faces only *B centered on B faces only *C centered on C faces only *R rhombohedral A reflection plane m within the point groups can be replaced by a glide plane, labeled as a, b, or c depending on which axis the glide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystal Structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystal, crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric patterns that repeat along the principal directions of Three-dimensional space (mathematics), three-dimensional space in matter. The smallest group of particles in the material that constitutes this repeating pattern is the unit cell of the structure. The unit cell completely reflects the symmetry and structure of the entire crystal, which is built up by repetitive Translation (geometry), translation of the unit cell along its principal axes. The translation vectors define the nodes of the Bravais lattice. The lengths of the principal axes, or edges, of the unit cell and the angles between them are the lattice constants, also called ''lattice parameters'' or ''cell parameters''. The symmetry properties of the crystal are described by the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the sublimation curve, fusion curve and the vaporisation curve meet. For example, the triple point of mercury occurs at a temperature of and a pressure of 0.165 m Pa. In addition to the triple point for solid, liquid, and gas phases, a triple point may involve more than one solid phase, for substances with multiple polymorphs. Helium-4 is a special case that presents a triple point involving two different fluid phases (lambda point). The triple point of water was used to define the kelvin, the base unit of thermodynamic temperature in the International System of Units (SI). The value of the triple point of water was fixed by definition, rather than measured, but that changed with the 2019 redefinition of SI base units. The triple points of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice VII
Ice VII is a cubic crystalline form of ice. It can be formed from liquid water above 3 GPa (30,000 atmospheres) by lowering its temperature to room temperature, or by decompressing heavy water (D2O) ice VI below 95 K. (Different types of ice, from ice II to ice XVIII, have been created in the laboratory at different temperatures and pressures. Ordinary water ice is known as ice Ih in the Bridgman nomenclature.) Ice VII is metastable over a wide range of temperatures and pressures and transforms into low-density amorphous ice (LDA) above . Ice VII has a triple point with liquid water and ice VI at 355 K and 2.216 GPa, with the melt line extending to at least and 10 GPa. Ice VII can be formed within nanoseconds by rapid compression via shock-waves. It can also be created by increasing the pressure on ice VI at ambient temperature. At around 5 GPa, Ice VII becomes the tetragonal Ice VIIt. Like the majority of ice phases (including ice Ih), the hydrogen atom positions are dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ice V
Ice V, pronounced "ice five", is a monoclinic crystalline phase of water, formed by cooling water to 253 K at 500 MPa. It has a density of 1.24 g cm3 (at 350 MPa). Ice V has a complicated structure, including 4-membered, 5-membered, 6-membered, and 8-membered rings and a total of 28 molecules A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioche ... in the unit cell. Ganymede's interior probably includes a liquid water ocean with tens to hundreds of kilometers of ice V at its base. References Water ice {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |