|
Imino Acid
In organic chemistry, an imino acid is any molecule that contains both imine (>C=NH) and carboxyl (-C(=O)-OH) functional groups. Imino acids are structurally related to amino acids, which have amino group instead of imine—a difference of single vs double-bond between nitrogen and carbon. The simplest example is dehydroglycine. D-Amino acid oxidase is an enzyme that is able to convert amino acids into imino acids. Also the direct biosynthetic precursor to the amino acid proline is the imino acid (''S'')-Δ1-pyrroline-5-carboxylate (P5C). Related terminology Secondary amino acids, amino acids containing a secondary amine group are sometimes named imino acids, though this usage is obsolescent. The only proteinogenic amino acid of this type is proline, although the related non-proteinogenic amino acids hydroxyproline and pipecolic acid Pipecolic acid (piperidine-2-carboxylic acid) is an organic compound with the formula HNC5H9CO2H. It is a carboxylic acid derivativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Delta-1-pyrroline-5-carboxylic Acid
1-Pyrroline-5-carboxylic acid (systematic name 3,4-dihydro-2H-pyrrole-2-carboxylic acid) is a cyclic imino acid. Its conjugate base and anion is 1-pyrroline-5-carboxylate (P5C). In solution, P5C is in spontaneous Equilibrium chemistry, equilibrium with Glutamate-5-semialdehyde, glutamate-5-semialdhyde (GSA). The chirality (chemistry), stereoisomer (''S'')-1-pyrroline-5-carboxylate (also referred to as L-P5C) is an intermediate metabolite in the biosynthesis and degradation of proline and arginine. In Prokaryote, prokaryotic proline biosynthesis, GSA is synthesized from γ-glutamyl phosphate by the enzyme γ-glutamyl phosphate reductase. In most eukaryotes, GSA is synthesised from the amino acid glutamate by the bifunctional enzyme 1-pyrroline-5-carboxylate synthase (P5CS). The human P5CS is encoded by the ''Aldehyde dehydrogenase 18 family, member A1, ALDH18A1'' gene. The enzyme pyrroline-5-carboxylate reductase converts P5C into proline In proline degradation, the enzyme proline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthetic
Biosynthesis is a multi-step, enzyme-Catalysis, catalyzed process where substrate (chemistry), substrates are converted into more complex Product (chemistry), products in living organisms. In biosynthesis, simple Chemical compound, compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: Precursor (chemistry), precursor compounds, chemical energy (e.g. adenosine triphosphate, ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some imp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pipecolic Acid
Pipecolic acid (piperidine-2-carboxylic acid) is an organic compound with the formula HNC5H9CO2H. It is a carboxylic acid derivative of piperidine and, as such, an amino acid, although not one encoded genetically. Like many other α-amino acids, pipecolic acid is chiral, although the S-stereoisomer is more common. It is a colorless solid. Its biosynthesis starts from lysine. CRYM, a taxon-specific protein that also binds thyroid hormones, is involved in the pipecolic acid pathway. Medicine It accumulates in pipecolic acidemia. Pipecolic acid can be associated with some forms of epilepsy. Occurrence and reactions Like most amino acids, pipecolic acid is a chelating agent. One complex is Cu(HNC5H9CO2)2(H2O)2. Pipecolic acid was identified in the Murchison meteorite. It also occurs in the leaves of the genus ''Myroxylon'', a tree from South America. See also * Bupivacaine * Efrapeptin Efrapeptins are peptides produced by fungi in the genus ''Tolypocladium'' that have antifunga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
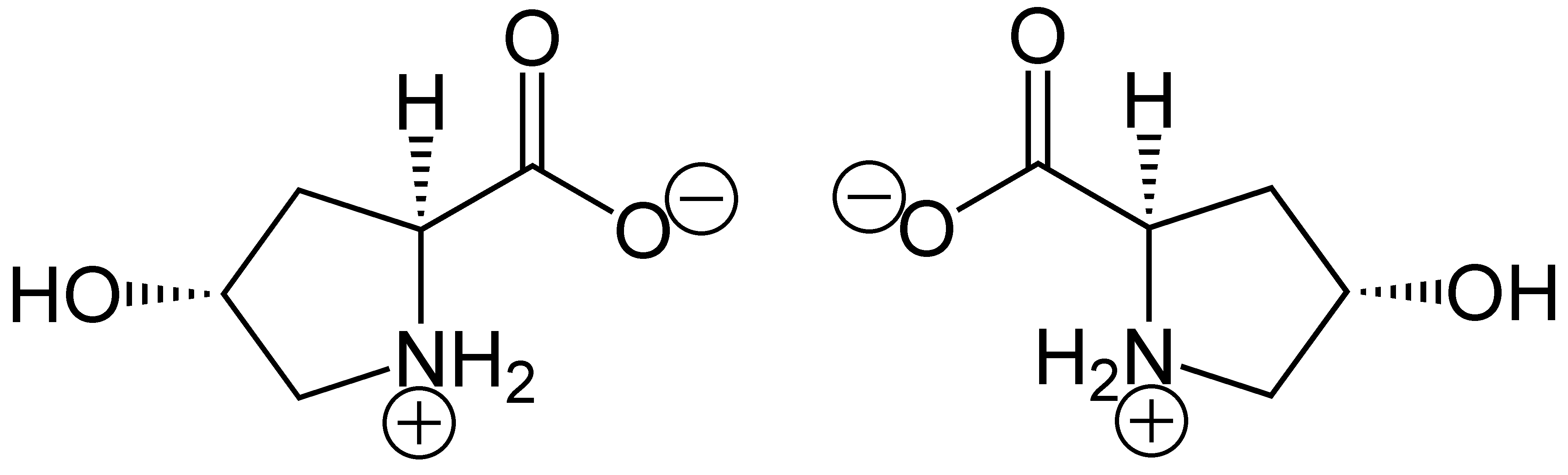
Hydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank. Structure and discovery In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelatin. In 1905, Hermann Leuchs synthesized a racemic mixture of 4-hydroxyproline. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom. Production and function Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl hydroxylase following protein synthesis (as a post-translational modification). The enzyme catalyzed reaction takes place in the lumen of the endoplasmic reticulum. Although it is not directly incorporated into proteins, hydroxyproline comprises roughly 4% of all amino acids found in animal tissue, an amount greater than seven other amino acids that are translationally incorporated. Animals Collagen Hydroxyproline is a major compon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-proteinogenic Amino Acids
In biochemistry, non-coded or non-proteinogenic amino acids are distinct from the 22 proteinogenic amino acids (21 in eukaryotesplus formylmethionine in eukaryotes with prokaryote organelles like mitochondria) which are naturally encoded in the genome of organisms for the assembly of proteins. However, over 140 non-proteinogenic amino acids occur naturally in proteins and thousands more may occur in nature or be synthesized in the laboratory. Chemically synthesized amino acids can be called unnatural amino acids. Unnatural amino acids can be synthetically prepared from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backbone. Many non-proteinogenic amino acids are important: * intermediates in biosynthesis, * in post-translational formation of proteins, * in a physiological role (e.g. components of bacterial cell walls, neurotransmitters and toxins), * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteinogenic Amino Acid
Proteinogenic amino acids are amino acids that are incorporated biosynthetically into proteins during translation. The word "proteinogenic" means "protein creating". Throughout known life, there are 22 genetically encoded (proteinogenic) amino acids, 20 in the standard genetic code and an additional 2 ( selenocysteine and pyrrolysine) that can be incorporated by special translation mechanisms. In contrast, non-proteinogenic amino acids are amino acids that are either not incorporated into proteins (like GABA, L-DOPA, or triiodothyronine), misincorporated in place of a genetically encoded amino acid, or not produced directly and in isolation by standard cellular machinery (like hydroxyproline). The latter often results from post-translational modification of proteins. Some non-proteinogenic amino acids are incorporated into nonribosomal peptides which are synthesized by non-ribosomal peptide synthetases. Both eukaryotes and prokaryotes can incorporate selenocysteine into their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Amino Acid
Secondary amino acids are amino acids which do not contain the amino group but is rather a secondary amine. Secondary amino acids can be classified to cyclic acids such as proline and acyclic N-substituted amino acids. In nature, proline, hydroxyproline, pipecolic acid and sarcosine are well-known secondary amino acids. Proline is the only proteinogenic secondary amino acids. Other secondary amino acids are non-proteinogenic amino acids. In protein, hydroxyproline is incorporated into protein by hydroxylation of proline. Pipecolic acid, a heavier analog of proline, is found in efrapeptin. Sarcosine is a N-methylized glycine so its methyl group is used in many biochemical reactions. Azetidine-2-carboxylic acid, which is a smaller homolog of proline in plants. Properties Proline and its higher homolog pipecolic acid affect the secondary structure of protein. D-alpha-amino acid - L-alpha-amino acid sequence can induce beta hairpin. It suggested that acyclic secondary amino acids a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |