|
Imido
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand. Nomenclature Most imides are cyclic compounds derived from dicarboxylic acids, and their names reflect the parent acid. Examples are succinimide, derived from succinic acid, and phthalimide, derived from phthalic acid. For imides derived from amines (as opposed to ammonia), the ''N''-substituent is indicated by a prefix. For example, N-ethylsuccinimide is derived from succinic acid and ethylamine. Isoimides are isomeric with normal imides and have the formula RC(O)OC(NR′)R″. They are often intermediates that convert to the more symmetr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Imide
The inorganic imides are compounds containing an ion composed of nitrogen bonded to hydrogen with formula HN2−. Organic imides have the NH group, and two single or one double covalent bond to other atoms. The imides are related to the inorganic amides (H2N−), the nitrides (N3−) and the nitridohydrides (N3−•H−). In addition to solid state imides, molecular imides are also known in dilute gases, where their spectrum can be studied. Imide can be a ligand, with a double bond to a metal such as molybdenum (e.g. Mo=NH). As a ligand it is called imido. The imido ligand is part of a nitrogen fixation cycle: Mo•N2 → Mo-N=N− → Mo-N=NH (diazenido) → Mo-N=NH2+ → Mo=N-NH2 (hydrazido) → Mo=N-NH3+ (hydrazidium) → Mo≡N (nitrido) + NH3 → Mo≡NH+ → Mo=NH (imido) → Mo=NH2+ → Mo-NH2 (amido) → Mo-NH3+ → Mo•NH3 (ammine); with the oxidation state of molybdenum varying to accommodate the number bonds from nitrogen. When the hydrogen of the imide group is sub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imide Functional Group
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand. Nomenclature Most imides are cyclic compounds derived from dicarboxylic acids, and their names reflect the parent acid. Examples are succinimide, derived from succinic acid, and phthalimide, derived from phthalic acid. For imides derived from amines (as opposed to ammonia), the ''N''-substituent is indicated by a prefix. For example, N-ethylsuccinimide is derived from succinic acid and ethylamine. Isoimides are isomeric with normal imides and have the formula RC(O)OC(NR′)R″. They are often intermediates that convert to the more symmet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phthalimide
Phthalimide is the organic compound with the formula C6H4(CO)2NH. It is the imide derivative of phthalic anhydride. It is a sublimable white solid that is slightly soluble in water but more so upon addition of base. It is used as a precursor to other organic compounds as a masked source of ammonia. Preparation Phthalimide can be prepared by heating phthalic anhydride with alcoholic ammonia giving 95–97% yield. Alternatively, it may be prepared by treating the anhydride with ammonium carbonate or urea. It can also be produced by ammoxidation of ''o''-xylene. Uses Phthalimide is used as a precursor to anthranilic acid, a precursor to azo dyes and saccharin. Alkyl phthalimides are useful precursors to amines in chemical synthesis, especially in peptide synthesis where they are used "to block both hydrogens and avoid racemization of the substrates". Alkyl halides can be converted to the N-alkylphthalimide: : C6H4(CO)2NH + RX + NaOH → C6H4(CO)2NR + NaX + H2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thalidomide
Thalidomide, sold under the brand names Contergan and Thalomid among others, is a medication used to treat a number of cancers (including multiple myeloma), graft-versus-host disease, and a number of skin conditions including complications of leprosy. While it has been used in a number of HIV-associated conditions, such use is associated with increased levels of the virus. It is taken by mouth. Common side effects include sleepiness, rash, and dizziness. Severe side effects include tumor lysis syndrome, blood clots, and peripheral neuropathy. Use in pregnancy may harm the fetus, including resulting in malformation of the limbs. In males who are taking the medication, contraception is essential if a partner could become pregnant. It is an immunomodulatory medication and works by a number of mechanisms, including stimulating T cells and decreasing TNF-α production. Thalidomide was first marketed in 1957 in West Germany, where it was available over the counter. Whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
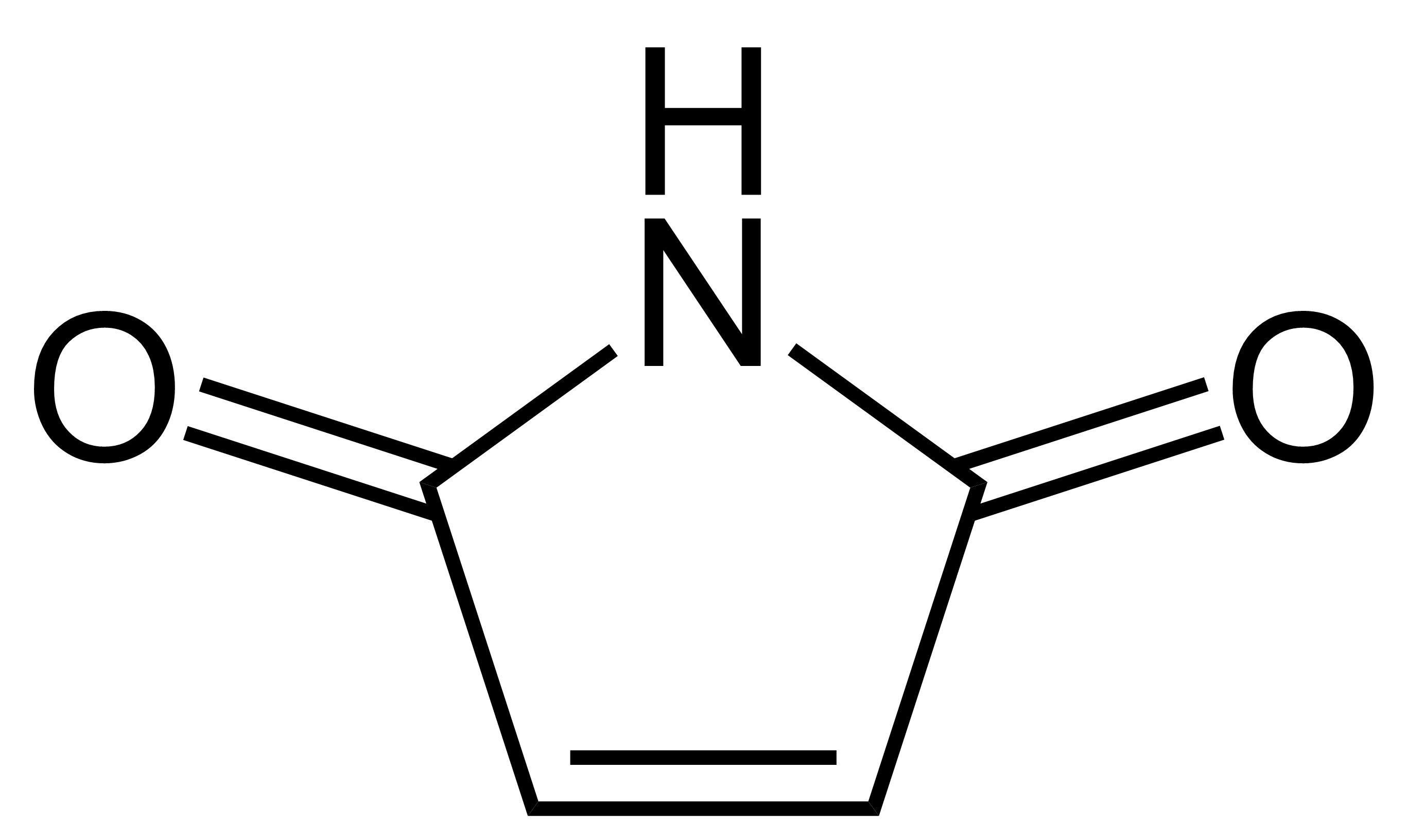
Maleimide
Maleimide is a chemical compound with the formula H2C2(CO)2NH (see diagram). This unsaturated imide is an important building block in organic synthesis. The name is a contraction of maleic acid and imide, the -C(O)NHC(O)- functional group. Maleimides also describes a ''class'' of derivatives of the parent maleimide where the N''H'' group is replaced with alkyl or aryl groups such as a methyl or phenyl, respectively. The substituent can also be a small molecule (such as biotin, a fluorescent dye, an oligosaccharide, or a nucleic acid), a reactive group, or a synthetic polymer such as polyethylene glycol. Human hemoglobin chemically modified with maleimide-polyethylene glycol is a blood substitute called MP4. Organic chemistry Maleimide and its derivatives are prepared from maleic anhydride by treatment with amines followed by dehydration. A special feature of the reactivity of maleimides is their susceptibility to additions across the double bond either by Michael additions or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their nonpolar core of carbon atoms and thus add chemical character to carbon chains. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyimide
Polyimide (sometimes abbreviated PI) is a polymer containing imide groups belonging to the class of high-performance plastics. With their high heat-resistance, polyimides enjoy diverse applications in roles demanding rugged organic materials, e.g. high temperature fuel cells, displays, and various military roles. A classic polyimide is Kapton, which is produced by condensation of pyromellitic dianhydride and 4,4'-oxydianiline.Wright, Walter W. and Hallden-Abberton, Michael (2002) "Polyimides" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. History The first polyimide was discovered in 1908 by Bogart and Renshaw. They found that 4-amino phthalic anhydride does not melt when heated but does release water upon the formation of a high molecular weight polyimide. The first semialiphatic polyimide was prepared by Edward and Robinson by melt fusion of diamines and tetra acids or diamines and diacids / diester. However, the first polyimide of significant co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutaric Acid
Glutaric acid is the organic compound with the formula C3H6(COOH)2 . Although the related "linear" dicarboxylic acids adipic and succinic acids are water-soluble only to a few percent at room temperature, the water-solubility of glutaric acid is over 50% (w/w). Biochemistry Glutaric acid is naturally produced in the body during the metabolism of some amino acids, including lysine and tryptophan. Defects in this metabolic pathway can lead to a disorder called glutaric aciduria, where toxic byproducts build up and can cause severe encephalopathy. Production Glutaric acid can be prepared by the ring-opening of butyrolactone with potassium cyanide to give the mixed potassium carboxylate- nitrile that is hydrolyzed to the diacid. Alternatively hydrolysis, followed by oxidation of dihydropyran gives glutaric acid. It can also be prepared from reacting 1,3-dibromopropane with sodium or potassium cyanide to obtain the dinitrile, followed by hydrolysis. Uses *1,5-Pentanediol, a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, and profit. Fungicides are used both in agriculture and to fight fungal infections in animals. Chemicals used to control oomycetes, which are not fungi, are also referred to as fungicides, as oomycetes use the same mechanisms as fungi to infect plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upwardly. Most fungicides that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloheximide
Cycloheximide is a naturally occurring fungicide produced by the bacterium '' Streptomyces griseus''. Cycloheximide exerts its effects by interfering with the translocation step in protein synthesis (movement of two tRNA molecules and mRNA in relation to the ribosome), thus blocking eukaryotic translational elongation. Cycloheximide is widely used in biomedical research to inhibit protein synthesis in eukaryotic cells studied ''in vitro'' (''i.e.'' outside of organisms). It is inexpensive and works rapidly. Its effects are rapidly reversed by simply removing it from the culture medium. Due to significant toxic side effects, including DNA damage, teratogenesis, and other reproductive effects (including birth defects and toxicity to sperm), cycloheximide is generally used only in ''in vitro'' research applications, and is not suitable for human use as a therapeutic compound. Although it has been used as a fungicide in agricultural applications, this application is now decre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kapton
Structure of poly-oxydiphenylene-pyromellitimide Kapton insulating pads for mounting electronic parts on a heat sink Kapton is a polyimide film used in flexible printed circuits ( flexible electronics) and space blankets, which are used on spacecraft, satellites, and various space instruments. Invented by the DuPont Corporation in the 1960s, Kapton remains stable (in isolation) across a wide range of temperatures, from . History Kapton was invented by the DuPont Corporation in the 1960s. The name ''Kapton'' is a registered trademark of E. I. du Pont de Nemours and Company. Chemistry and variants Kapton synthesis is an example of the use of a dianhydride in step polymerization. The intermediate polymer, known as a ''poly(amic acid)'', is soluble because of strong hydrogen bonds to the polar solvents usually employed in the reaction. The ring closure is carried out at high temperatures of . The chemical name for Kapton K and HN is ''poly (4,4'-oxydiphenylene-pyrome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)
