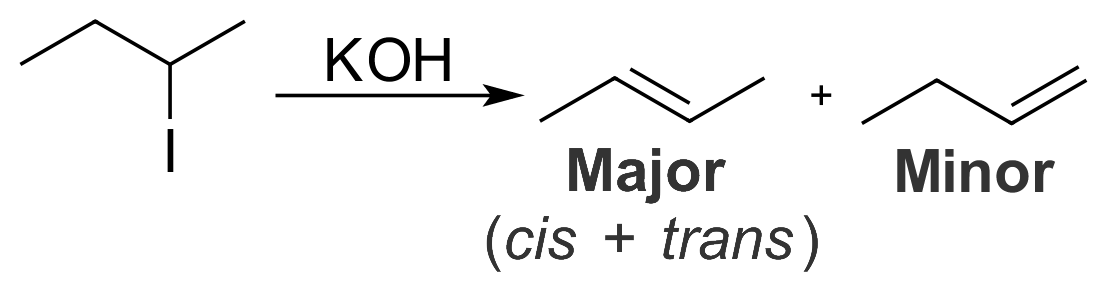|
Hyper-conjugation
In organic chemistry, hyperconjugation (σ-conjugation or no-bond resonance) refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electrons in a sigma (σ) orbital (e.g. C–H or C–C) with an adjacent unpopulated non-bonding p or antibonding σ* or π* orbitals to give a pair of extended molecular orbitals. However, sometimes, low-lying antibonding σ* orbitals may also interact with filled orbitals of lone pair character (n) in what is termed '' negative hyperconjugation''. Increased electron delocalization associated with hyperconjugation increases the stability of the system. In particular, the new orbital with bonding character is stabilized, resulting in an overall stabilization of the molecule. Only electrons in bonds that are in the β position can have this sort of direct stabilizing effect — donating from a sigma bond on an atom to an orbital in anothe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-silicon Effect
The beta-silicon effect also called silicon hyperconjugation in organosilicon chemistry is a special type of hyperconjugation that describes the stabilizing influence of a silicon atom on the development of positive charge at a carbon atom one position removed (β) from the silicon atom. The C-Si σ orbital is said to partially overlap with the σ* anti-bonding orbital of the C-leaving group, lowering the energy of the transition state leading to the formation of a carbocation. A prerequisite for the hyperconjugation to occur is an antiperiplanar relationship between the Si group and the leaving group.''Silicon in Organic Synthesis'' Colvin, E. Butterworth: London 1981 This allows for the maximum overlap between the C-Si σ orbital and the σ* anti-bonding orbital of the leaving group. Silicon hyperconjugation explains specific observations regarding chemical kinetics and stereochemistry of organic reactions with reactants containing silicon. The picture below shows the partial ov ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma Bonds
In chemistry, sigma bonds (σ bonds) are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is symmetrical with respect to rotation about the bond axis. By this definition, common forms of sigma bonds are s+s, pz+pz, s+pz and dz2+dz2 (where z is defined as the axis of the bond or the internuclear axis). Quantum theory also indicates that molecular orbitals (MO) of identical symmetry actually mix or ''hybridize''. As a practical consequence of this mixing of diatomic molecules, the wavefunctions s+s and pz+pz molecular orbitals become blended. The extent of this mixing (or hybridization or blending) depends on the relative energies of the MOs of like symmetry. For homodiatomics (homonuclear diatomic molecules), bonding σ orbitals have no nodal planes at which the wavefunction is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Explanation Bond length is related to bond order: when more electrons participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors being equal, a stronger bond will be shorter. In a bond between two identical atoms, half the bond distance is equal to the covalent radius. Bond lengths are measured in the solid phase by means of X-ray diffraction, or approximated in the gas phase by microwave spectroscopy. A bond between a given pair of atoms may vary between different molecules. For example, the carbon to hydrogen bonds in methane are different from those in methyl chloride. It is however possible to make generalizations when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron (journal)
''Tetrahedron'' is a weekly peer-reviewed scientific journal covering the field of organic chemistry. According to the ''Journal Citation Reports'', ''Tetrahedron'' has a 2020 impact factor of 2.457. ''Tetrahedron'' and Elsevier, its publisher, support an annual symposium. In 2010, complaints were raised over its high subscription cost. Notable papers , the Web of Science lists ten papers from ''Tetrahedron'' that have more than 1000 citations. The four articles that have been cited more than 2000 times are: * – cited 2228 times * – cited 2162 times * – cited 2124 times * – cited 2107 times See also * ''Tetrahedron Letters'' * ''Tetrahedron Computer Methodology'' * ''Polyhedron In geometry, a polyhedron (plural polyhedra or polyhedrons; ) is a three-dimensional shape with flat polygonal faces, straight edges and sharp corners or vertices. A convex polyhedron is the convex hull of finitely many points, not all on th ...'' (journal) Refere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" * Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel *CHEM-DT CHEM-DT is the TVA owned-and-operated television station in Trois-Rivières, Quebec, Canada. It broadcasts a high-definition digital signal on VHF channel 8 from a transmitter on Rue Principale in Notre-Dame-du-Mont-Carmel. Owned by the Grou ..., a Canadian television channel See also * Chemo (other) * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nature (journal)
''Nature'' is a British weekly scientific journal founded and based in London, England. As a multidisciplinary publication, ''Nature'' features peer-reviewed research from a variety of academic disciplines, mainly in science and technology. It has core editorial offices across the United States, continental Europe, and Asia under the international scientific publishing company Springer Nature. ''Nature'' was one of the world's most cited scientific journals by the Science Edition of the 2019 ''Journal Citation Reports'' (with an ascribed impact factor of 42.778), making it one of the world's most-read and most prestigious academic journals. , it claimed an online readership of about three million unique readers per month. Founded in autumn 1869, ''Nature'' was first circulated by Norman Lockyer and Alexander Macmillan as a public forum for scientific innovations. The mid-20th century facilitated an editorial expansion for the journal; ''Nature'' redoubled its efforts in exp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Effects
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staggered Conformation
In organic chemistry, a staggered conformation is a chemical conformation of an ethane-like Moiety (chemistry), moiety abcX–Ydef in which the substituents a, b, and c are at the maximum distance from d, e, and f; this requires the torsion angles to be 60°. It is the opposite of an eclipsed conformation, in which those substituents are as close to each other as possible. Such a conformation exists in any open chain single chemical bond connecting two sp3-orbital hybridisation, hybridised atoms, and is normally a conformational energy minimum. For some molecules such as those of ''n''-butane, there can be special versions of staggered conformations called ''gauche'' and ''anti''; see first Newman projection diagram in Conformational isomerism. Crystal structures: The staggered/eclipsed configurations distinguish different crystalline structures of e.g. cubic/hexagonal boron nitride, and diamond/lonsdaleite. See also * Alkane stereochemistry * Eclipsed conformation References ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zaitsev's Rule
In organic chemistry, Zaitsev's rule (or Saytzeff's rule, Saytzev's rule) is an empirical rule for predicting the favored alkene product(s) in elimination reactions. While at the University of Kazan, Russian chemist Alexander Zaitsev studied a variety of different elimination reactions and observed a general trend in the resulting alkenes. Based on this trend, Zaitsev proposed that the alkene formed in greatest amount is that which corresponded to removal of the hydrogen from the alpha-carbon having the fewest hydrogen substituents. For example, when 2-iodobutane is treated with alcoholic potassium hydroxide (KOH), 2-butene is the major product and 1-butene is the minor product. : More generally, Zaitsev's rule predicts that in an elimination reaction the most substituted product will be the most stable, and therefore the most favored. The rule makes no generalizations about the stereochemistry of the newly formed alkene, but only the regiochemistry of the elimination reaction. Wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountered (e.g., ethylene dication ). Until the early 1970s, all carbocations were called ''carbonium ions''. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further classified in two main categories according to the coordination number of the charged carbon: three in the carbenium ions and five in the carbonium ions. This nomenclature was proposed by G. A. Olah. Carbonium ions, as originally defined by Olah, are characterized by a three-center two-electron delocalized bonding scheme and are essentially synonymous with so-called 'non-classical carbocations', which are carbocations that contain bridging C–C or C–H σ-bonds. Howe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |