|
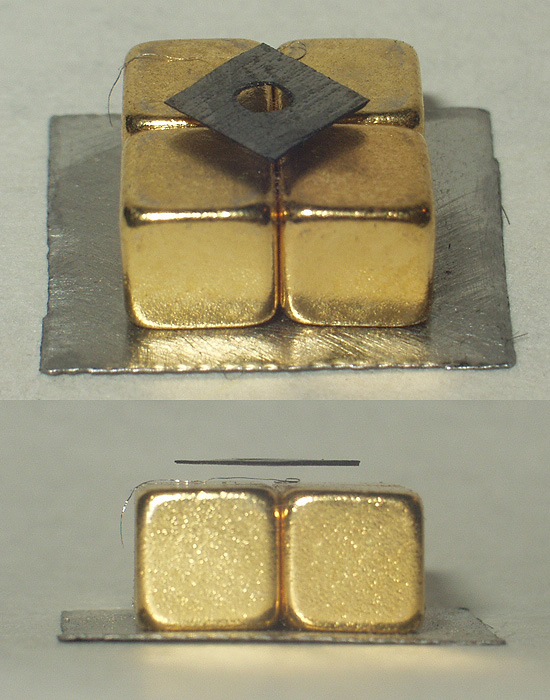
Hafnium(IV) Carbide
Hafnium carbide ( Hf C) is a chemical compound of hafnium and carbon. Previously the material was estimated to have a melting point of about 3,900 °C. More recent tests have been able to conclusively prove that the substance has an even higher melting point of 3,958 °C exceeding those of tantalum carbide and tantalum hafnium carbide which were both previously estimated to be higher. However, it has a low oxidation resistance, with the oxidation starting at temperatures as low as 430 °C. Experimental testing in 2018 confirmed the higher melting point yielding a result of 3,982 (±30°C) with a small possibility that the melting point may even exceed 4,000°C. Atomistic simulations conducted in 2015 predicted that a Hf-C-N material could have a melting point exceeding even that of hafnium carbide. More recent experimental evidence gathered in 2020 confirmed that hafnium carbonitride did indeed have a higher melting point exceeding 4,000 °C. Hafnium carbide i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the Crystal_structure#Unit_cell, unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called Close-packing_of_equal_spheres, ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive_cell, primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one Lattice_(group), lattice point on each corner of the cube; this means each simple cubic unit cell has in total one latt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high quality, and high-performance, solid materials. The process is often used in the semiconductor industry to produce thin films. In typical CVD, the wafer (substrate) is exposed to one or more volatile precursors, which react and/or decompose on the substrate surface to produce the desired deposit. Frequently, volatile by-products are also produced, which are removed by gas flow through the reaction chamber. Microfabrication processes widely use CVD to deposit materials in various forms, including: monocrystalline, polycrystalline, amorphous, and epitaxial. These materials include: silicon ( dioxide, carbide, nitride, oxynitride), carbon (fiber, nanofibers, nanotubes, diamond and graphene), fluorocarbons, filaments, tungsten, titanium nitride and various high-κ dielectrics. The term ''chemical vapour deposition'' was coined 1960 by ''John M. Blocher, Jr.'' who intended to differentiate ''chemic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium Compounds
Hafnium compounds are compounds containing the element hafnium (Hf). Due to the lanthanide contraction, the ionic radius of hafnium(IV) (0.78 ångström) is almost the same as that of zirconium(IV) (0.79 angstroms). Consequently, compounds of hafnium(IV) and zirconium(IV) have very similar chemical and physical properties. Hafnium and zirconium tend to occur together in nature and the similarity of their ionic radii makes their chemical separation rather difficult. Hafnium tends to form inorganic compounds in the oxidation state of +4. Halogens react with it to form hafnium tetrahalides. At higher temperatures, hafnium reacts with oxygen, nitrogen, carbon, boron, sulfur, and silicon. Some compounds of hafnium in lower oxidation states are known. Halides Hafnium(IV) fluoride (HfF4) is a white crystalline powder. It has a monoclinic crystal structure, with space group C2/c (No.15), and lattice constants ''a'' = 1.17 nm, ''b'' = 0.986 nm and ''c'' = 0.764 nm. Hafnium(IV) c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbides
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece. Interstitial / Metallic carbides The carbides of the group 4, 5 and 6 transition metals (with the exception of chromium) are often described as interstitial compounds. These carbides have metallic properties and are refractory. Some exhibit a range of stoichiometries, being a non-stoichiometric mixture of various carbides arising due to crystal defects. Some of them, including titanium carbide and tungsten carbide, are important industrially and are used to coat metals in cutting tools. The long-held view is that the carbon atoms fit into octahedral interstices in a close-packed metal lattice when the metal atom radius is greater than approximately 135 pm: *When the metal atoms are cubic close-packed, (ccp), then filling all of the octahedral interstices with carbon achieves 1:1 stoich ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hafnium Carbonitride
Hafnium carbonitride (HfCN) is a mixed anion chemical compound of hafnium, carbon and nitrogen. With a melting point of above 4,000 °C, it is the most refractory compound known. No other substance has a higher melting point at atmospheric pressure. In 2015, atomistic simulations predicted that a Hf-C-N material could have a melting point exceeding Ta4Hf1C5 and hafnium carbide Hafnium carbide ( Hf C) is a chemical compound of hafnium and carbon. Previously the material was estimated to have a melting point of about 3,900 °C. More recent tests have been able to conclusively prove that the substance has an even hig ... by 200 °C. This would later be proven in experimental testing conducted in 2020 by the National University of Science and Technology (NUST) in Moscow. Samples of both hafnium carbide and hafnium carbonitride were tested in the same environment in which hafnium carbonitride was shown to have a melting point exceeding 4,000 °C and higher than that of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum Hafnium Carbide
Tantalum hafnium carbide is a refractory chemical compound with a general formula , which can be considered as a solid solution of tantalum carbide and hafnium carbide. Properties Individually, tantalum and hafnium carbide have the highest melting points among the binary compounds, and , respectively, and their "alloy" with a composition Ta4HfC5 has an melting point of . Very few measurements of melting point in tantalum hafnium carbide have been reported, because of the obvious experimental difficulties at extreme temperatures. A 1965 study of the TaC-HfC solid solutions at temperatures 2,225–2,275 °C found a minimum in the vaporization rate and thus maximum in the thermal stability for Ta4HfC5. This rate was comparable to that of tungsten and was weakly dependent on the initial density of the samples, which were sintered from TaC-HfC powder mixtures, also at 2,225–2,275 °C. In a separate study, Ta4HfC5 was found to have the minimum oxidation rate among the Ta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Springer Science%2BBusiness Media
Springer Science+Business Media, commonly known as Springer, is a German multinational publishing company of books, e-books and peer-reviewed journals in science, humanities, technical and medical (STM) publishing. Originally founded in 1842 in Berlin, it expanded internationally in the 1960s, and through mergers in the 1990s and a sale to venture capitalists it fused with Wolters Kluwer and eventually became part of Springer Nature in 2015. Springer has major offices in Berlin, Heidelberg, Dordrecht, and New York City. History Julius Springer founded Springer-Verlag in Berlin in 1842 and his son Ferdinand Springer grew it from a small firm of 4 employees into Germany's then second largest academic publisher with 65 staff in 1872.Chronology ". Springer Science+Business Media. In 1964, Springer expanded its business internationally, o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tantalum Carbide
Tantalum carbides (TaC) form a family of binary chemical compounds of tantalum and carbon with the empirical formula TaC''x'', where ''x'' usually varies between 0.4 and 1. They are extremely hard, brittle, refractory ceramic materials with metallic electrical conductivity. They appear as brown-gray powders, which are usually processed by sintering. Being important cermet materials, tantalum carbides are commercially used in tool bits for cutting applications and are sometimes added to tungsten carbide alloys. The melting points of tantalum carbides was previously estimated to be about 3,880 °C depending on the purity and measurement conditions; this value is among the highest for binary compounds. And only tantalum hafnium carbide was estimated to have a higher melting point of 3,942 °C. However new tests have conclusively proven that TaC actually has a melting point of 3,768 °C and both tantalum hafnium carbide and hafnium carbide have higher melting points. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior. Diamagnetism was first discovered when Anton Brugmans observed in 1778 that bismuth was repel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, diamagnetic materials are repelled by magnetic fields and form induced magnetic fields in the direction opposite to that of the applied magnetic field. Paramagnetic materials include most chemical elements and some compounds; they have a relative magnetic permeability slightly greater than 1 (i.e., a small positive magnetic susceptibility) and hence are attracted to magnetic fields. The magnetic moment induced by the applied field is linear in the field strength and rather weak. It typically requires a sensitive analytical balance to detect the effect and modern measurements on paramagnetic materials are often conducted with a SQUID magnetometer. Paramagnetism is due to the presence of unpaired electrons in the material, so most atoms wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Academic Press
Academic Press (AP) is an academic book publisher founded in 1941. It was acquired by Harcourt, Brace & World in 1969. Reed Elsevier bought Harcourt in 2000, and Academic Press is now an imprint of Elsevier. Academic Press publishes reference books, serials and online products in the subject areas of: * Communications engineering * Economics * Environmental science * Finance * Food science and nutrition * Geophysics * Life sciences * Mathematics and statistics * Neuroscience * Physical sciences * Psychology Well-known products include the ''Methods in Enzymology'' series and encyclopedias such as ''The International Encyclopedia of Public Health'' and the ''Encyclopedia of Neuroscience''. See also * Akademische Verlagsgesellschaft (AVG) — the German predecessor, founded in 1906 by Leo Jolowicz (1868–1940), the father of Walter Jolowicz Walter may refer to: People * Walter (name), both a surname and a given name * Little Walter, American blues harmonica player Marion Wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Press
The CRC Press, LLC is an American publishing group that specializes in producing technical books. Many of their books relate to engineering, science and mathematics. Their scope also includes books on business, forensics and information technology. CRC Press is now a division of Taylor & Francis, itself a subsidiary of Informa. History The CRC Press was founded as the Chemical Rubber Company (CRC) in 1903 by brothers Arthur, Leo and Emanuel Friedman in Cleveland, Ohio, based on an earlier enterprise by Arthur, who had begun selling rubber laboratory aprons in 1900. The company gradually expanded to include sales of laboratory equipment to chemists. In 1913 the CRC offered a short (116-page) manual called the ''Rubber Handbook'' as an incentive for any purchase of a dozen aprons. Since then the ''Rubber Handbook'' has evolved into the CRC's flagship book, the '' CRC Handbook of Chemistry and Physics''. In 1964, Chemical Rubber decided to focus on its publishing ventures ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_oxide.jpg)