|
Group 15 Hydride
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, and bismuth) covalently bonded to hydrogen. Pnictogen trihydrides The simplest series has the chemical formula XH3 (less commonly H3X), with X representing any of the pnictogens. They take on the pyramidal structure (as opposed to the trigonal planar arrangement of the group 13 hydrides), and therefore are polar. These pnictogen trihydrides are generally increasingly unstable and poisonous with heavier elements. Like the simple hydrogen halides and chalcogenides, the pnictogen hydrides are water- soluble. Unlike other hydrides such as hydrogen sulfide and hydrogen fluoride, which form acidic aqueous solutions, ammonia dissolves in water to make ammonium hydroxide which is basic (by forming a hydroxide ion as opposed to hydronium). Phosphine is also water-s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binary Compounds Of Hydrogen
Binary compounds of hydrogen are binary compound, binary chemical compounds containing just hydrogen and one other chemical element. By convention all binary hydrogen compounds are called hydrides even when the hydrogen atom in it is not an anion. These hydrogen compounds can be grouped into several types. Overview Binary hydrogen compounds in group 1 element, group 1 are the ionic hydrides (also called saline hydrides) wherein hydrogen is bound electrostatically. Because hydrogen is located somewhat centrally in an electronegative sense, it is necessary for the counterion to be exceptionally electropositive for the hydride to possibly be accurately described as truly behaving ionic. Therefore, this category of hydrides contains only a few members. Hydrides in group 2 element, group 2 are polymeric covalent hydrides. In these, hydrogen forms bridging covalent bonds, usually possessing mediocre degrees of ionic character, which make them difficult to be accurately described as either ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with discoveri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
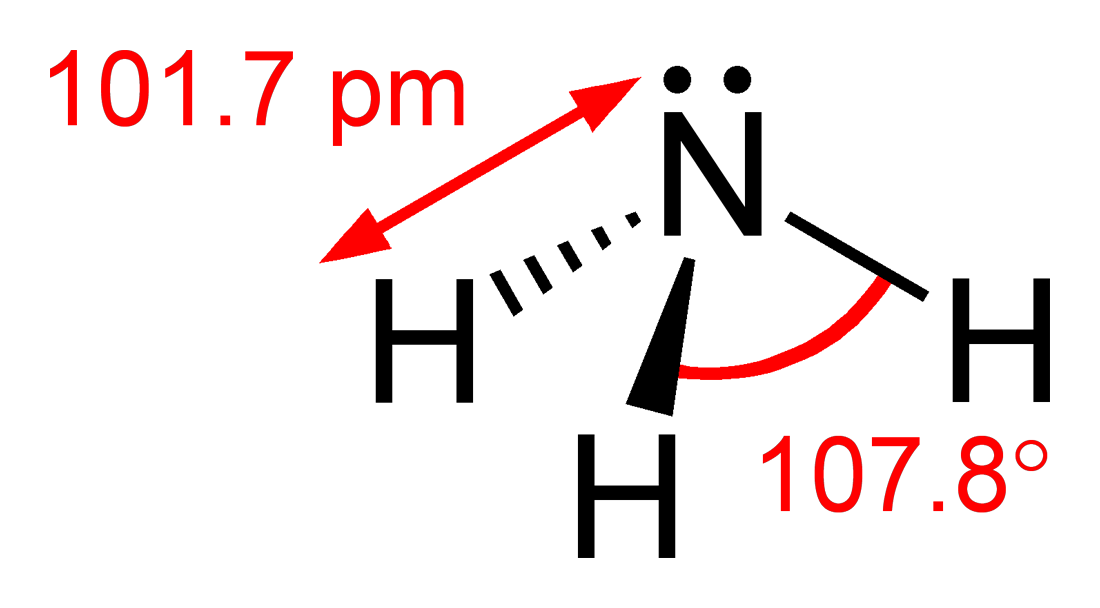
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuthine
Bismuthine (IUPAC name: bismuthane) is the chemical compound with the formula BiH3. As the heaviest analogue of ammonia (a pnictogen hydride), BiH3 is unstable, decomposing to bismuth metal well below 0 °C. This compound adopts the expected pyramidal structure with H–Bi–H angles of around 90°. The term ''bismuthine'' may also refer to a member of the family of organobismuth(III) species having the general formula , where R is an organic substituent. For example, Bi(CH3)3 is ''trimethylbismuthine''. Preparation and properties BiH3 is prepared by the redistribution of methylbismuthine (BiH2Me):Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001.. :3 BiH2Me → 2 BiH3 + BiMe3 The required BiH2Me, which is also thermally unstable, is generated by reduction of methylbismuth dichloride, BiCl2Me with LiAlH4. As suggested by the behavior of SbH3, BiH3 is unstable and decomposes to its constituent elements according to the following equa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stibine
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). This gas has an offensive smell like hydrogen sulfide (rotten eggs). Preparation SbH3 is generally prepared by the reaction of Sb3+ sources with H− equivalents: :2 Sb2O3 + 3 LiAlH4 → 4 SbH3 + 1.5 Li2O + 1.5 Al2O3 :4 SbCl3 + 3 NaBH4 → 4 SbH3 + 3 NaCl + 3 BCl3 Alternatively, sources of Sb3− react with protonic reagents (even water) to also produce this unstable gas: :Na3Sb + 3 H2O → SbH3 + 3 NaOH Properties The chemical properties of SbH3 resemble those for AsH3. Typical for a heavy hydride (e.g. AsH3, H2Te, SnH4), SbH3 is unstable with respect to its elements. The gas decomposes slowly at room temperature but rapidly at 200 °C: ::2 SbH3 → 3 H2 + 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term ''arsine'' is commonly used to describe a class of organoarsenic compounds of the formula AsH3−xRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine". General properties At its standard state, arsine is a colorless, denser-than-air gas that is slightly soluble in water (20% at 20 °C) and in many organic solvents as well. Whereas arsine itself is odorless, owing to its oxidation by air, it is possible to smell a slight garlic or fish-like scent when the compound is present above 0.5 ppm. This compound is kinetically stable: at room temperature it decomposes only slowly. At temperatures of ca. 230&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in water, as Arrhenius acid molecules in solution give up a proton (a positive hydrogen ion, ) to the surrounding water molecules (). In fact, acids must be surrounded by more than a single water molecule in order to ionize, yielding aqueous and conjugate base. Three main structures for the aqueous proton have garnered experimental support: The Eigen cation, which is a tetrahydrate, H3O+(H2O)3; the Zundel cation, which is a symmetric dihydrate, H+(H2O)2; and the Stoyanov cation, an expanded Zundel cation, which is a hexahydrate: H+(H2O)2(H2O)4. Spectroscopic evidence from well-defined IR spectra overwhelmingly supports the Stoyanov cation as the predominant form. For this reason, it has been suggested that wherever possible ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |