|
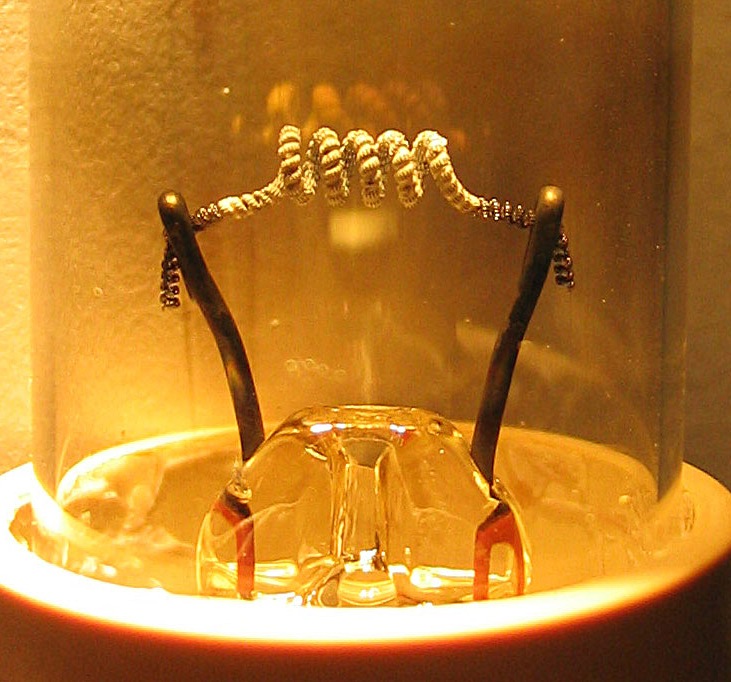
Glow Switch Starter
A glow switch starter or glowbottle starter is a type of preheat starter used with a fluorescent lamp. It is commonly filled with neon gas or argon gas and contains a bimetallic strip and a stationary electrode. The operating principle is simple, when current is applied, the gas inside ionizes and heats a bimetallic strip which in turn bends toward the stationary electrode thus shorting the starter between the electrodes of the fluorescent lamp. After about a second the starter's bimetallic strip cools and opens the circuit between the electrodes, and the process repeats until the lamp has lit. One disadvantage of glow switch starters is that when the lamp is at the end of its life it will continuously blink on and off until the glow switch starter wears out or an electrode on the fluorescent lamp burns out. Glow starters have a relatively short life, and light fittings enable the starter to be changed easily. Fluorescent lamp#Preheating, Electronic starters, being interchangeable ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Various Types Of Glow Starter
Various may refer to: * Various (band), an English dubstep/electronic music duo * Various artists, a term for a compilation album containing pieces by various musicians * Various authors, a book containing works by several writers * ''The Various'', a children's fantasy novel by Steve Augarde See also * Various & Gould, a Berlin-based artist duo * ''Various Artists – Archives Vol. 4'', an album by Steve Vai * ''Various Failures'', a compilation album by American experimental rock band Swans * ''The Various Haunts of Men'', a novel by Susan Hill * ''Various Positions'', an album by Leonard Cohen ** Various Positions Tour * Various Positions (film), ''Various Positions'' (film), a 2002 film directed by Ori Kowarsky * Varius (other) * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet light that then causes a phosphor coating on the inside of the lamp to glow. A fluorescent lamp converts electrical energy into useful light much more efficiently than an incandescent lamp. The typical luminous efficacy of fluorescent lighting systems is 50–100 lumens per watt, several times the efficacy of incandescent bulbs with comparable light output. For comparison, the luminous efficacy of an incandescent bulb may only be 16 lumens per watt. Fluorescent lamp fixtures are more costly than incandescent lamps because, among other things, they require a ballast to regulate current through the lamp, but the initial cost is offset by a much lower running cost. Compact fluorescent lamps are now available in the same popular sizes as incand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neon
Neon is a chemical element with the symbol Ne and atomic number 10. It is a noble gas. Neon is a colorless, odorless, inert monatomic gas under standard conditions, with about two-thirds the density of air. It was discovered (along with krypton and xenon) in 1898 as one of the three residual rare inert elements remaining in dry air, after nitrogen, oxygen, argon and carbon dioxide were removed. Neon was the second of these three rare gases to be discovered and was immediately recognized as a new element from its bright red emission spectrum. The name neon is derived from the Greek word, , neuter singular form of (), meaning 'new'. Neon is chemically inert, and no uncharged neon compounds are known. The compounds of neon currently known include ionic molecules, molecules held together by van der Waals forces and clathrates. During cosmic nucleogenesis of the elements, large amounts of neon are built up from the alpha-capture fusion process in stars. Although neon is a very co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element with the symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third-most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all of the argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete octe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bimetallic Strip
A bimetallic strip is used to convert a temperature change into mechanical displacement. The strip consists of two strips of different metals which expand at different rates as they are heated. The different expansions force the flat strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled. The invention of the bimetallic strip is generally credited to John Harrison, an eighteenth-century clockmaker who made it for his third marine chronometer (H3) of 1759 to compensate for temperature-induced changes in the balance spring. Harrison's invention is recognized in the memorial to him in Westminster Abbey, England. This effect is used in a range of mechanical and electrical devices. Characteristics The strip consists of two strips of different metals which expand at different rates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery. The electrophore, invented by Johan Wilcke, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery made was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes separated by brine-soaked paper disks. Due to fluctuation in the voltage provided by the voltaic cell it wasn't very practical. The first practical battery was invented in 1839 and named the Daniell cell after John Frederic Daniell. Still making use of the zinc–copper electrode combination. Since then many more batteries have be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Lamp
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet light that then causes a phosphor coating on the inside of the lamp to glow. A fluorescent lamp converts electrical energy into useful light much more efficiently than an incandescent lamp. The typical luminous efficacy of fluorescent lighting systems is 50–100 lumens per watt, several times the efficacy of incandescent bulbs with comparable light output. For comparison, the luminous efficacy of an incandescent bulb may only be 16 lumens per watt. Fluorescent lamp fixtures are more costly than incandescent lamps because, among other things, they require a ballast to regulate current through the lamp, but the initial cost is offset by a much lower running cost. Compact fluorescent lamps are now available in the same popular sizes as incand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent Light
A fluorescent lamp, or fluorescent tube, is a low-pressure mercury-vapor gas-discharge lamp that uses fluorescence to produce visible light. An electric current in the gas excites mercury vapor, which produces short-wave ultraviolet light that then causes a phosphor coating on the inside of the lamp to glow. A fluorescent lamp converts electrical energy into useful light much more efficiently than an incandescent lamp. The typical luminous efficacy of fluorescent lighting systems is 50–100 lumens per watt, several times the efficacy of incandescent bulbs with comparable light output. For comparison, the luminous efficacy of an incandescent bulb may only be 16 lumens per watt. Fluorescent lamp fixtures are more costly than incandescent lamps because, among other things, they require a ballast to regulate current through the lamp, but the initial cost is offset by a much lower running cost. Compact fluorescent lamps are now available in the same popular sizes as incand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glow Discharge
A glow discharge is a plasma formed by the passage of electric current through a gas. It is often created by applying a voltage between two electrodes in a glass tube containing a low-pressure gas. When the voltage exceeds a value called the striking voltage, the gas ionization becomes self-sustaining, and the tube glows with a colored light. The color depends on the gas used. Glow discharges are used as a source of light in devices such as neon lights, fluorescent lamps, and plasma-screen televisions. Analyzing the light produced with spectroscopy can reveal information about the atomic interactions in the gas, so glow discharges are used in plasma physics and analytical chemistry. They are also used in the surface treatment technique called sputtering. Electrical conduction in gas Conduction in a gas requires charge carriers, which can be either electrons or ions. Charge carriers come from ionizing some of the gas molecules. In terms of current flow, glow discharge falls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bimetallic Strip
A bimetallic strip is used to convert a temperature change into mechanical displacement. The strip consists of two strips of different metals which expand at different rates as they are heated. The different expansions force the flat strip to bend one way if heated, and in the opposite direction if cooled below its initial temperature. The metal with the higher coefficient of thermal expansion is on the outer side of the curve when the strip is heated and on the inner side when cooled. The invention of the bimetallic strip is generally credited to John Harrison, an eighteenth-century clockmaker who made it for his third marine chronometer (H3) of 1759 to compensate for temperature-induced changes in the balance spring. Harrison's invention is recognized in the memorial to him in Westminster Abbey, England. This effect is used in a range of mechanical and electrical devices. Characteristics The strip consists of two strips of different metals which expand at different rates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermionic Emission
Thermionic emission is the liberation of electrons from an electrode by virtue of its temperature (releasing of energy supplied by heat). This occurs because the thermal energy given to the charge carrier overcomes the work function of the material. The charge carriers can be electrons or ions, and in older literature are sometimes referred to as thermions. After emission, a charge that is equal in magnitude and opposite in sign to the total charge emitted is initially left behind in the emitting region. But if the emitter is connected to a battery, the charge left behind is neutralized by charge supplied by the battery as the emitted charge carriers move away from the emitter, and finally the emitter will be in the same state as it was before emission. The classical example of thermionic emission is that of electrons from a hot cathode into a vacuum (also known as thermal electron emission or the Edison effect) in a vacuum tube. The hot cathode can be a metal filament, a coated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Short-circuit
A short circuit (sometimes abbreviated to short or s/c) is an electrical circuit that allows a current to travel along an unintended path with no or very low electrical impedance. This results in an excessive current flowing through the circuit. The opposite of a short circuit is an "open circuit", which is an infinite resistance between two nodes. Definition A short circuit is an abnormal connection between two nodes of an electric circuit intended to be at different voltages. This results in an electric current limited only by the Thévenin equivalent resistance of the rest of the network which can cause circuit damage, overheating, fire or explosion. Although usually the result of a fault, there are cases where short circuits are caused intentionally, for example, for the purpose of voltage-sensing crowbar circuit protectors. In circuit analysis, a ''short circuit'' is defined as a connection between two nodes that forces them to be at the same voltage. In an 'ideal' sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |