|
Gas Centrifuges
A gas centrifuge is a device that performs isotope separation of gases. A centrifuge relies on the principles of centrifugal force accelerating molecules so that particles of different masses are physically separated in a gradient along the radius of a rotating container. A prominent use of gas centrifuges is for the separation of uranium-235 (235U) from uranium-238 (238U). The gas centrifuge was developed to replace the gaseous diffusion method of uranium-235 extraction. High degrees of separation of these isotopes relies on using many individual centrifuges arranged in series, that achieve successively higher concentrations. This process yields higher concentrations of uranium-235 while using significantly less energy compared to the gaseous diffusion process. Centrifugal process The centrifuge relies on the force resulting from centrifugal acceleration to separate molecules according to their mass, and can be applied to most fluids. The dense (heavier) molecules move towards th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Countercurrent Gas Centrifuge
Countercurrent may refer to: *Countercurrent pool *Countercurrent exchange *Countercurrent chromatography *Equatorial Counter Current *''Counter-Currents'', an alt-right online publication *''Countercurrents.org'', an Indian news website *two political party factions in Italy: **Countercurrent (PRC faction, Italy), a faction of the Communist Refoundation Party **Countercurrent (PdL faction, Italy), a faction of The People of Freedom See also *Counter currency, an element of a currency pair in foreign exchange *Against the Current (other) {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
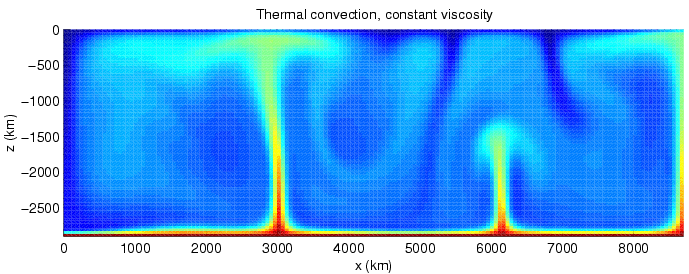
Convection Currents
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow. Convective flow may be transient (such as when a multiphase mixture of oil and water separates) or steady state (see Convection cell). The convection may be due to gravitational, electromagnetic or fictitious body forces. Heat transfer by natural convection plays a role in the structure of Earth's atmosphere, its oceans, and its mantle. Discrete convective cells in the atmosphere can be identified by clouds, with stronger convection resulting in thunderstorms. Natural convection also plays a role in stellar physics. Convection is often categorised or des ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Virginia
The University of Virginia (UVA) is a Public university#United States, public research university in Charlottesville, Virginia. Founded in 1819 by Thomas Jefferson, the university is ranked among the top academic institutions in the United States, with College admissions in the United States, highly selective admission. Set within the The Lawn, Academical Village, a World Heritage Site, UNESCO World Heritage Site, the university is referred to as a "Public Ivy" for offering an academic experience similar to that of an Ivy League university. It is known in part for certain rare characteristics among public universities such as #1800s, its historic foundations, #Honor system, student-run academic honor code, honor code, and Secret societies at the University of Virginia, secret societies. The original governing Board of Visitors included three List of presidents of the United States, U.S. presidents: Thomas Jefferson, Jefferson, James Madison, and James Monroe. The latter as si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jesse Beams
Jesse Wakefield Beams (December 25, 1898 in Belle Plaine, Kansas – July 23, 1977) was an American physicist at the University of Virginia. Biography Beams completed his undergraduate B.A. in physics at Fairmount College in 1921 and his master's degree the next year at the University of Wisconsin. He spent most of his academic career at the University of Virginia, where he received his Ph.D. in physics in 1925. He spent the next three years in a physics fellowship at Yale University, where he performed research on the photoelectric effect with Ernest Lawrence. Beams was appointed a professor of physics at the University of Virginia in 1929 and was chair of the department from 1948 to 1962. During World War II, he worked on the Manhattan Project, where his ultracentrifuge was used to demonstrate the separation of the lighter uranium isotope U-235 from other isotopes. Officials in charge of the atomic bomb project concluded, however, that Beams's centrifuges were not as likely ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion Inhibitor
In chemistry, a corrosion inhibitor or anti-corrosive is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy, that comes into contact with the fluid. The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime. Corrosion inhibitors are common in industry, and also found in over-the-counter products, typically in spray form in combination with a lubricant and sometimes a penetrating oil. They may be added to water to prevent leaching of lead or copper from pipes. A common mechanism for inhibiting corrosion involves formation of a coating, often a passivation layer, which prevents access of the corrosive substance to the metal. Permanent treatments such as chrome plating are not generally considered inhibitors, however: corrosion inhibitors are additives to the fluids that surround the metal or related object. Types The nature of the corrosive inhibitor de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depleted Zinc Oxide
Depleted zinc oxide (DZO) is a zinc oxide depleted in the zinc isotope with the atomic mass 64, and used as a corrosion inhibitor in nuclear pressurized water reactors. The depletion of 64Zn is necessary, because this isotope is transformed into 65Zn by neutron capture. 65Zn with a half-life of 244.26 days emits gamma radiation with 1.115 MeV. 64Zn has a natural abundance of 48.6%, but in DZO it is reduced below 1%. Adding zinc oxide to the primary water loop of a boiling water reactor or pressurized water nuclear reactor reduces corrosion and therefore minimizes the amount of dissolved materials, especially 60Co. The isotope separation of zinc is done by gas centrifugation of diethylzinc Diethylzinc (C2H5)2Zn, or DEZ, is a highly pyrophoric and reactive organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry. It is available commercially as a .... References * External links * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Zinc
Diethylzinc (C2H5)2Zn, or DEZ, is a highly pyrophoric and reactive organozinc compound consisting of a zinc center bound to two ethyl groups. This colourless liquid is an important reagent in organic chemistry. It is available commercially as a solution in hexanes, heptane, or toluene, or as a pure liquid. Synthesis Edward Frankland first reported the compound in 1848 from zinc and ethyl iodide, the first organozinc compound discovered. He improved the synthesis by using diethyl mercury as starting material. The contemporary synthesis consists of the reaction of a 1:1 mixture of ethyl iodide and ethyl bromide with a zinc-copper couple, a source of reactive zinc. Structure The compound crystallizes in a tetragonal body-centered unit cell of space group symmetry I41md. In the solid-state diethylzinc shows nearly linear Zn centres. The Zn-C bonds measure 194.8(5) pm, while the C-Zn-C angle is slightly bent with 176.2(4)°. The structure of the gas-phase shows a very similar Zn-C dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products, and neutrons (in nuclear fission). Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years. Neutron activation is the only common way that a stable material can be induced into becoming intrinsically radioactive. All naturally occurring materials, including air, water, and soil, can be induced (activated) by neutron capture into some amount of radioactivity in varying degrees, as a result of the production of neutron-rich radioisotopes. Some atoms require more than one neu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (''t''1/2) for t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc-64
Naturally occurring zinc (30Zn) is composed of the 5 stable isotopes 64Zn, 66Zn, 67Zn, 68Zn, and 70Zn with 64Zn being the most abundant (48.6% natural abundance). Twenty-five radioisotopes have been characterised with the most abundant and stable being 65Zn with a half-life of 244.26 days, and 72Zn with a half-life of 46.5 hours. All of the remaining radioactive isotopes have half-lives that are less than 14 hours and the majority of these have half-lives that are less than 1 second. This element also has 10 meta states. Zinc has been proposed as a " salting" material for nuclear weapons. A jacket of isotopically enriched 64Zn, irradiated by the intense high-energy neutron flux from an exploding thermonuclear weapon, would transmute into the radioactive isotope 65Zn with a half-life of 244 days and produce approximately 1.115 MeV of gamma radiation, significantly increasing the radioactivity of the weapon's fallout for several years. Such a weapon is not known to have ever b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranium Enrichment
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U with 99.2739–99.2752% natural abundance), uranium-235 (235U, 0.7198–0.7202%), and uranium-234 (234U, 0.0050–0.0059%). 235U is the only nuclide existing in nature (in any appreciable amount) that is fissile with thermal neutrons. Enriched uranium is a critical component for both civil nuclear power generation and military nuclear weapons. The International Atomic Energy Agency attempts to monitor and control enriched uranium supplies and processes in its efforts to ensure nuclear power generation safety and curb nuclear weapons proliferation. There are about 2,000 tonnes of highly enriched uranium in the world, produced mostly for nuclear power, nuclear weapons, naval propulsion, and smaller quantities for research reactors. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |