|
Gelling
A gel is a semi-solid that can have properties ranging from soft and weak to hard and tough. Gels are defined as a substantially dilute cross-linked system, which exhibits no flow when in the steady-state, although the liquid phase may still diffuse through this system. A gel has been defined phenomenologically as a soft, solid or solid-like material consisting of two or more components, one of which is a liquid, present in substantial quantity. By weight, gels are mostly liquid, yet they behave like solids because of a three-dimensional cross-linked network within the liquid. It is the crosslinking within the fluid that gives a gel its structure (hardness) and contributes to the adhesive stick (tack). In this way, gels are a dispersion of molecules of a liquid within a solid medium. The word ''gel'' was coined by 19th-century Scottish chemist Thomas Graham by clipping from '' gelatine''. The process of forming a gel is called gelation. IUPAC definition } ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gelatine
Gelatin or gelatine (from la, gelatus meaning "stiff" or "frozen") is a translucent, colorless, flavorless food ingredient, commonly derived from collagen taken from animal body parts. It is brittle when dry and rubbery when moist. It may also be referred to as hydrolyzed collagen, collagen hydrolysate, gelatine hydrolysate, hydrolyzed gelatine, and collagen peptides after it has undergone hydrolysis. It is commonly used as a gelling agent in food, beverages, medications, drug or vitamin capsules, photographic films, papers, and cosmetics. Substances containing gelatin or functioning in a similar way are called gelatinous substances. Gelatin is an irreversibly hydrolyzed form of collagen, wherein the hydrolysis reduces protein fibrils into smaller peptides; depending on the physical and chemical methods of denaturation, the molecular weight of the peptides falls within a broad range. Gelatin is present in gelatin desserts, most gummy candy and marshmallows, ice creams, dips, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rheology
Rheology (; ) is the study of the flow of matter, primarily in a fluid (liquid or gas) state, but also as "soft solids" or solids under conditions in which they respond with plastic flow rather than deforming elastically in response to an applied force. Rheology is a branch of physics, and it is the science that deals with the deformation and flow of materials, both solids and liquids.W. R. Schowalter (1978) Mechanics of Non-Newtonian Fluids Pergamon The term '' rheology'' was coined by Eugene C. Bingham, a professor at Lafayette College, in 1920, from a suggestion by a colleague, Markus Reiner.The Deborah Number The term was inspired by the of |
Gelation
In polymer chemistry, gelation (gel transition) is the formation of a gel from a system with polymers. Branched polymers can form links between the chains, which lead to progressively larger polymers. As the linking continues, larger branched polymers are obtained and at a certain extent of the reaction links between the polymer result in the formation of a single macroscopic molecule. At that point in the reaction, which is defined as gel point, the system loses fluidity and viscosity becomes very large. The onset of gelation, or gel point, is accompanied by a sudden increase in viscosity. This "infinite" sized polymer is called the gel or network, which does not dissolve in the solvent, but can swell in it. Background Gelation is promoted by gelling agents. Gelation can occur either by physical linking or by chemical crosslinking. While the physical gels involve physical bonds, chemical gelation involves covalent bonds. The first quantitative theories of chemical gelation w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word '' suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study was introduced in 1845 b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Percolation Theory
In statistical physics and mathematics, percolation theory describes the behavior of a network when nodes or links are added. This is a geometric type of phase transition, since at a critical fraction of addition the network of small, disconnected clusters merge into significantly larger connected, so-called spanning clusters. The applications of percolation theory to materials science and in many other disciplines are discussed here and in the articles network theory and percolation. Introduction A representative question (and the source of the name) is as follows. Assume that some liquid is poured on top of some porous material. Will the liquid be able to make its way from hole to hole and reach the bottom? This physical question is modelled mathematically as a three-dimensional network of vertices, usually called "sites", in which the edge or "bonds" between each two neighbors may be open (allowing the liquid through) with probability , or closed with probability , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
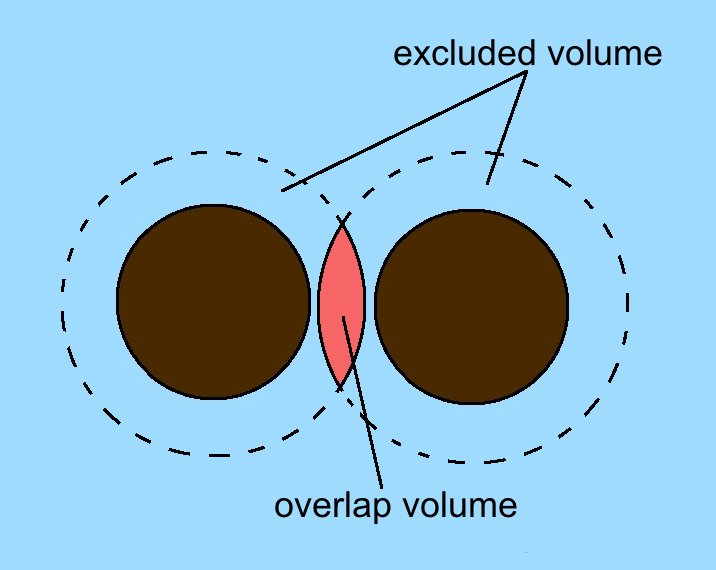
Depletion Force
A depletion force is an effective attractive force that arises between large colloidal particles that are suspended in a dilute solution of ''depletants'', which are smaller solutes that are preferentially excluded from the vicinity of the large particles. One of the earliest reports of depletion forces that lead to particle coagulation is that of Bondy, who observed the separation or "creaming" of rubber latex upon addition of polymer depletant molecules ( sodium alginate) to solution. More generally, depletants can include polymers, micelles, osmolytes, ink, mud, or paint dispersed in a continuous phase. Depletion forces are often regarded as entropic forces, as was first explained by the established Asakura–Oosawa model. In this theory the depletion force arises from an increase in osmotic pressure of the surrounding solution when colloidal particles get close enough such that the excluded cosolutes (depletants) cannot fit in between them. Because the particles were conside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption (chemistry)
In chemistry, absorption is a physical or chemical phenomenon or a process in which atoms, molecules or ions enter some bulk phase – liquid or solid material. This is a different process from adsorption, since molecules undergoing absorption are taken up by the volume, not by the surface (as in the case for adsorption). A more common definition is that "Absorption is a chemical or physical phenomenon in which the molecules, atoms and ions of the substance getting absorbed enters into the bulk phase (gas, liquid or solid) of the material in which it is taken up." A more general term is '' sorption'', which covers absorption, adsorption, and ion exchange. Absorption is a condition in which something takes in another substance. In many processes important in technology, the chemical absorption is used in place of the physical process, e.g., absorption of carbon dioxide by sodium hydroxide – such acid-base processes do not follow the Nernst partition law (see: solubility). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Smart Materials
Smart materials, also called intelligent or responsive materials, are designed materials that have one or more properties that can be significantly changed in a controlled fashion by external stimuli, such as stress, moisture, electric or magnetic fields, light, temperature, pH, or chemical compounds. Smart materials are the basis of many applications, including sensors and actuators, or artificial muscles, particularly as electroactive polymers (EAPs). Terms used to describe smart materials include shape memory material (SMM) and shape memory technology (SMT). Types There are a number of types of smart material, of which are already common. Some examples are as following: * Piezoelectric materials are materials that produce a voltage when stress is applied. Since this effect also applies in a reverse manner, a voltage across the sample will produce stress within sample. Suitably designed structures made from these materials can, therefore, be made that bend, expand or cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |