|
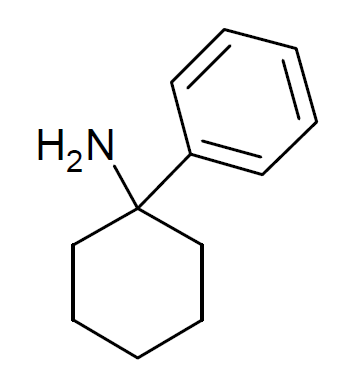
Fourphit
Fourphit (4-isothiocyanato-1- -phenylcyclohexyliperidine) is a covalent binding NMDA antagonist, dopaminergic and sigma receptor agonist. See also * Metaphit Metaphit (1- -(3-Isothiocyanato)phenylyclohexylpiperidine) is a research chemical that acts as an acylator of NMDARAn, sigma and DAT binding sites in the CNS. It is the ''m''-isothiocyanate derivative of phencyclidine (PCP) and binds irreversi ... References Arylcyclohexylamines Dopamine reuptake inhibitors Dissociative drugs NMDA receptor antagonists Piperidines Sigma agonists Isothiocyanates {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metaphit
Metaphit (1- -(3-Isothiocyanato)phenylyclohexylpiperidine) is a research chemical that acts as an acylator of NMDARAn, sigma and DAT binding sites in the CNS. It is the ''m''-isothiocyanate derivative of phencyclidine (PCP) and binds irreversibly (forming a covalent bond) to the PCP binding site on the NMDA receptor complex. However, later studies suggest the functionality of metaphit is mediated by sites not involved in PCP-induced passive avoidance deficit, and not related to the NMDA receptor complex. Metaphit was also shown to prevent d-amphetamine induced hyperactivity, while significantly depleting dopamine content in the nucleus accumbens. Metaphit was the first acylating ligand used to study the cocaine receptor. It is a structural isomer of the similar research compound fourphit, as it and metaphit both are isothiocyanate substituted derivatives of an analogous scaffold shared with PCP. References {{Navboxes , title = Pharmacodynamics Pharmacodynamics (PD) is t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share " valence", such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMDA Antagonist
NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the ''N''-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce is referred to as dissociative anesthesia. Several synthetic opioids function additionally as NMDAR-antagonists, such as pethidine, levorphanol, methadone, dextropropoxyphene, tramadol and ketobemidone. Some NMDA receptor antagonists, such as ketamine, dextromethorphan (DXM), phencyclidine (PCP), methoxetamine (MXE), and nitrous oxide (N2O), are sometimes used as recreational drugs, for their dissociative, hallucinogenic, and euphoriant properties. When used recreationally, they are classified as dissociative drugs. Uses and effects NMDA receptor antagonists induce a state called dissociative anesthesia, marked by catalepsy, amnesia, and analgesia. Ketamine is a favored anesthetic for emergency patients with unknown medical history and i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopaminergic
Dopaminergic means "related to dopamine" (literally, "working on dopamine"), dopamine being a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain. Dopaminergic brain pathways facilitate dopamine-related activity. For example, certain proteins such as the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2), and dopamine receptors can be classified as dopaminergic, and neurons that synthesize or contain dopamine and synapses with dopamine receptors in them may also be labeled as ''dopaminergic''. Enzymes that regulate the biosynthesis or metabolism of dopamine such as aromatic L-amino acid decarboxylase or DOPA decarboxylase, monoamine oxidase (MAO), and catechol ''O''-methyl transferase (COMT) may be referred to as ''dopaminergic'' as well. Also, any endogenous or exogenous chemical substance that acts to affect dopamine receptors or dopamine release through indirect actions (for example, on neurons t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma Receptor
Sigma receptors (σ-receptors) are protein cell surface receptors that bind ligands such as 4-PPBP (4-phenyl-1-(4-phenylbutyl) piperidine), SA 4503 (cutamesine), ditolylguanidine, dimethyltryptamine, and siramesine. There are two subtypes, sigma-1 receptors (σ1) and sigma-2 receptors (σ2), which are classified as sigma receptors for their pharmacological similarities, even though they are evolutionarily unrelated. The fungal protein ERG2, a C-8 sterol isomerase, falls into the same protein family as sigma-1. Both localize to the ER membrane, although sigma-1 is also reported to be a cell surface receptor. Sigma-2 is an EXPREA domain protein (citation needed) with a mostly intracellular (ER membrane) localization. Classification Because the σ-receptor was originally discovered to be agonized by benzomorphan opioids and antagonized by naltrexone, σ-receptors were originally believed to be a type of opioid receptor. When the σ1 receptor was isolated and cloned, it was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmacodynamics
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs (especially pharmaceutical drugs). The effects can include those manifested within animals (including humans), microorganisms, or combinations of organisms (for example, infection). Pharmacodynamics and pharmacokinetics are the main branches of pharmacology, being itself a topic of biology interested in the study of the interactions between both endogenous and exogenous chemical substances with living organisms. In particular, pharmacodynamics is the study of how a drug affects an organism, whereas pharmacokinetics is the study of how the organism affects the drug. Both together influence dosing, benefit, and adverse effects. Pharmacodynamics is sometimes abbreviated as PD and pharmacokinetics as PK, especially in combined reference (for example, when speaking of PK/PD models). Pharmacodynamics places particular emphasis on dose–response relationships, that is, the relationships between d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arylcyclohexylamines
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 synthesis of phencyclidine and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to their dissociative hallucinogenic and euphoriant effects. Since that time, the class has be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine Reuptake Inhibitors
A dopamine reuptake inhibitor (DRI) is a class of drug which acts as a reuptake inhibitor of the monoamine neurotransmitter dopamine by blocking the action of the dopamine transporter (DAT). Reuptake inhibition is achieved when extracellular dopamine not absorbed by the postsynaptic neuron is blocked from re-entering the presynaptic neuron. This results in increased extracellular concentrations of dopamine and increase in dopaminergic neurotransmission. DRIs are used in the treatment of attention-deficit hyperactivity disorder (ADHD) and narcolepsy for their psychostimulant effects, and in the treatment of obesity and binge eating disorder for their appetite suppressant effects. They are sometimes used as antidepressants in the treatment of mood disorders, but their use as antidepressants is limited given that strong DRIs have a high abuse potential and legal restrictions on their use. Lack of dopamine reuptake and the increase in extracellular levels of dopamine have been linked ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociative Drugs
Dissociatives, colloquially dissos, are a subclass of hallucinogens which distort perception of sight and sound and produce feelings of detachment – dissociation – from the environment and/or self. Although many kinds of drugs are capable of such action, dissociatives are unique in that they do so in such a way that they produce hallucinogenic effects, which may include dissociation, a general decrease in sensory experience, hallucinations, dream-like states or anesthesia. Some of these substances, which are nonselective in action and affect the dopamine and/or opioid systems, may be capable of inducing euphoria or symptoms which are more akin to the effects of certain “hard drugs” or common drugs of abuse. This is likely why dissociatives are considered to be addictive with a fair to moderate potential for abuse, unlike psychedelics. Despite some dissociatives, such as phencyclidine (PCP) possessing stimulating properties, most dissociatives seem to have a general depre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMDA Receptor Antagonists
NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the ''N''-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce is referred to as dissociative anesthesia. Several synthetic opioids function additionally as NMDAR-antagonists, such as pethidine, levorphanol, methadone, dextropropoxyphene, tramadol and ketobemidone. Some NMDA receptor antagonists, such as ketamine, dextromethorphan (DXM), phencyclidine (PCP), methoxetamine (MXE), and nitrous oxide (N2O), are sometimes used as recreational drugs, for their dissociative, hallucinogenic, and euphoriant properties. When used recreationally, they are classified as dissociative drugs. Uses and effects NMDA receptor antagonists induce a state called dissociative anesthesia, marked by catalepsy, amnesia, and analgesia. Ketamine is a favored anesthetic for emergency patients with unknown medical history and i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidines
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name ''Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma Agonists
Sigma (; uppercase Σ, lowercase σ, lowercase in word-final position ς; grc-gre, σίγμα) is the eighteenth letter of the Greek alphabet. In the system of Greek numerals, it has a value of 200. In general mathematics, uppercase Σ is used as an operator for summation. When used at the end of a letter-case word (one that does not use all caps), the final form (ς) is used. In ' (Odysseus), for example, the two lowercase sigmas (σ) in the center of the name are distinct from the word-final sigma (ς) at the end. The Latin letter S derives from sigma while the Cyrillic letter Es derives from a lunate form of this letter. History The shape (Σς) and alphabetic position of sigma is derived from the Phoenician letter ( ''shin''). Sigma's original name may have been ''san'', but due to the complicated early history of the Greek epichoric alphabets, ''san'' came to be identified as a separate letter in the Greek alphabet, represented as Ϻ. Herodotus reports that "san" wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |