|
Formazan
The formazans are compounds of the general formula -N=N-C(R')=N-NH-R" formally derivatives of formazan 2NN=CHN=NH unknown in free form. Formazan dyes are artificial chromogenic products obtained by reduction of tetrazolium salts by dehydrogenases and reductases. They have a variety of colors from dark blue to deep red to orange, depending on the original tetrazolium salt used as the substrate for the reaction. Structure and reactivity Formazans are intensely colorful compounds characterized by the following structure: N=N-C(R)=N-NH- and are closely related to azo (−N=N−) dyes. Their structure was first defined in 1892, by von Pechmann and by Bamberger and Wheelwright independently. Their deep colour and redox chemistry derive from their nitrogen-rich backbone. Formazans have a high tautomeric and conformational flexibility. Due to the two alternating double bonds in the backbone, formazans can exist in four possible isomeric forms: syn, s-cis (closed form); syn, s-tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formazan
The formazans are compounds of the general formula -N=N-C(R')=N-NH-R" formally derivatives of formazan 2NN=CHN=NH unknown in free form. Formazan dyes are artificial chromogenic products obtained by reduction of tetrazolium salts by dehydrogenases and reductases. They have a variety of colors from dark blue to deep red to orange, depending on the original tetrazolium salt used as the substrate for the reaction. Structure and reactivity Formazans are intensely colorful compounds characterized by the following structure: N=N-C(R)=N-NH- and are closely related to azo (−N=N−) dyes. Their structure was first defined in 1892, by von Pechmann and by Bamberger and Wheelwright independently. Their deep colour and redox chemistry derive from their nitrogen-rich backbone. Formazans have a high tautomeric and conformational flexibility. Due to the two alternating double bonds in the backbone, formazans can exist in four possible isomeric forms: syn, s-cis (closed form); syn, s-tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formazans2Tetrazoles
The formazans are compounds of the general formula -N=N-C(R')=N-NH-R" formally derivatives of formazan 2NN=CHN=NH unknown in free form. Formazan dyes are artificial chromogenic products obtained by reduction of tetrazolium salts by dehydrogenases and reductases. They have a variety of colors from dark blue to deep red to orange, depending on the original tetrazolium salt used as the substrate for the reaction. Structure and reactivity Formazans are intensely colorful compounds characterized by the following structure: N=N-C(R)=N-NH- and are closely related to azo (−N=N−) dyes. Their structure was first defined in 1892, by von Pechmann and by Bamberger and Wheelwright independently. Their deep colour and redox chemistry derive from their nitrogen-rich backbone. Formazans have a high tautomeric and conformational flexibility. Due to the two alternating double bonds in the backbone, formazans can exist in four possible isomeric forms: syn, s-cis (closed form); syn, s-tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromogen
In chemistry, the term chromogen refers to a colourless (or faintly coloured) chemical compound that can be converted by chemical reaction into a compound which can be described as "coloured". There is no universally agreed definition of the term. Various dictionaries give the following definitions: * A substance capable of conversion into a pigment or dye. * Any substance that can become a pigment or coloring matter, a substance in organic fluids that forms colored compounds when oxidized, or a compound, not itself a dye, that can become a dye. * Any substance, itself without color, giving origin to a coloring matter. In biochemistry the term has a rather different meaning. The following are found in various dictionaries. * A precursor of a biochemical pigment * A pigment-producing microorganism * Any of certain bacteria that produce a pigment * A strongly pigmented or pigment-generating organelle, organ, or microorganism. Applications in chemistry *In chromogenic photography, f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelation
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a Denticity, polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast medium, contrast agents in MRI, MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoformate
In organic chemistry, an ortho ester is a functional group containing three alkoxy groups attached to one carbon atom, i.e. with the general formula . Orthoesters may be considered as products of exhaustive alkylation of unstable orthocarboxylic acids and it is from these that the name 'ortho ester' is derived. An example is ethyl orthoacetate, , more correctly known as 1,1,1-triethoxyethane. Synthesis Ortho esters can be prepared by the Pinner reaction, in which nitriles react with alcohols in the presence of one equivalent of hydrogen chloride. The reaction proceeds by formation of imido ester hydrochloride: :RCN + R′OH + HCl → C(OR′)=NH2sup>+Cl− Upon standing in the presence of excess alcohol, this intermediate converts to the ortho ester: : C(OR′)=NH2sup>+Cl− + 2R′OH → RC(OR′)3 + NH4Cl The reaction requires anhydrous conditions. Although a less common method, ortho esters were first produced by reaction of 1,1,1-trichloroalkanes with sodium alkoxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Formate
Ethyl formate is an ester formed when ethanol (an alcohol) reacts with formic acid (a carboxylic acid). Ethyl formate has the characteristic smell of rum and is also partially responsible for the flavor of raspberries. It occurs naturally in the body of ants and in the stingers of bees. Exposure Ethyl formate is generally recognized as safe by the U.S. Food and Drug Administration. According to the U.S Occupational Safety and Health Administration (OSHA), ethyl formate can irritate eyes, skin, mucous membranes, and the respiratory system of humans and other animals; it is also a central nervous system depressant. In industry, it is used as a solvent for cellulose nitrate, cellulose acetate, oils, and greases. It can be used as a substitute for acetone; workers may also be exposed to it under the following circumstances: * during spray, brush, or dip applications of lacquers * during the manufacture of safety glass * when fumigating tobacco, cereals, and dried fruits (as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
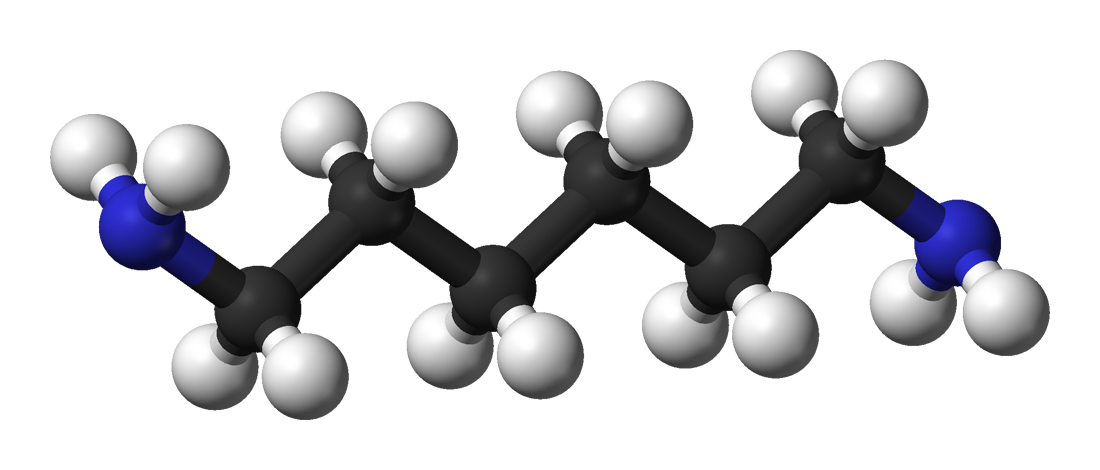
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydrazines r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrazene
Tetrazene is a chemical compound with the molecular formula H2NN=NNH2. It is a colorless explosive material. An analogue is the organosilicon derivative (tms)2NN=NN(tms)2 where tms is trimethylsilyl. Isomeric with tetrazine is ammonium azide. Tetrazene explosive, commonly known simply as tetrazene, is used for sensitization of priming compositions. Properties Tetrazene has eleven isomers. The most stable of these is the straight-chain 2-tetrazene (H2-NN=N-NH2), having a standard heat of formation at 301.3 kJ/mol. The eleven isomers can be arranged into three groups: straight-chain tetrazenes, four-membered cyclotetrazane, and three-membered cyclotriazanes. Each straight-chain tetrazene isomer possesses one N=N double bond and two N-N single bonds. Tautomerizations do occur between the isomers. The ionic compound ammonium azide is also a constitutional isomer of tetrazene. Organometallic derivatives A variety of coordination complex A coordination complex consists of a centr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by two single bonds. The group may be represented as , where the '<' denotes the two bonds. This can equally well be represented as . This stands in contrast to a situation where the carbon atom is bound to the rest of the molecule by a double bond, which is preferably called a , represented . Formerly the methylene name was used for both isomers. The name ““ can be used for the single-bonded isomer, to emphatically exclude methylidene. The distinction is often important, because the double bond is chemically di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. Synthesis Hydrazine, organohydrazines, and 1,1-diorganohydrazines react with aldehydes and ketones to give hydrazones. : Phenylhydrazine reacts with reducing sugars to form hydrazones known as osazones, which was developed by German chemist Emil Fischer as a test to differentiate monosaccharides. Uses Hydrazones are the basis for various analyses of ketones and aldehydes. For example, dinitrophenylhydrazine coated onto a silica sorbent is the basis of an adsorption cartridge. The hydrazones are then eluted and analyzed by HPLC using a UV detector. The compound carbonyl cyanide-''p''-trifluoromethoxyphenylhydrazone (abbreviated as FCCP) is used to uncouple ATP synthesis and reduction of oxygen in oxidative phosphorylation in molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres. Structure and bonding Aldehydes feature a carbon center that is connected by a double bond to oxygen and a single bond to hydrogen and single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The C=O bond length is about 120-122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes are more soluble in water, formaldehyde and acetaldehyde completely so. The volatile aldehydes have pungent odors. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diazonium Compound
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. General properties and reactivity Arenediazonium cations and related species According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group lowers the p''K''a (enhances the acidity) by a million-fold. The stabi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |