|
Foldase
In molecular biology, foldases are a particular kind of molecular chaperones that assist the non-covalent folding of proteins in an ATP-dependent manner. Examples of foldase systems are the GroEL/GroES and the DnaK/DnaJ/GrpE system. References {{reflist http://www.embl.de/pepcore/pepcore_services/protein_expression/ecoli/improving_protein_solubility/ See also * Holdase * Chaperonin * Co-chaperone Co-chaperones are proteins that assist chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly essential in stimulation ... Molecular chaperones Protein biosynthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holdase
In molecular biology, holdases are a particular kind of molecular chaperones that assist the non-covalent folding of proteins in an ATP-independent manner. Examples of holdases are DnaJ and Hsp33. Holdases bind to protein folding intermediates to prevent their aggregation but without directly refolding them. They stand in opposition to foldases, which are chaperones that use ATP to fold proteins. See also * Foldase * Chaperonin * Co-chaperone Co-chaperones are proteins that assist chaperone (protein), chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly esse ... References {{reflist Molecular chaperones Protein biosynthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
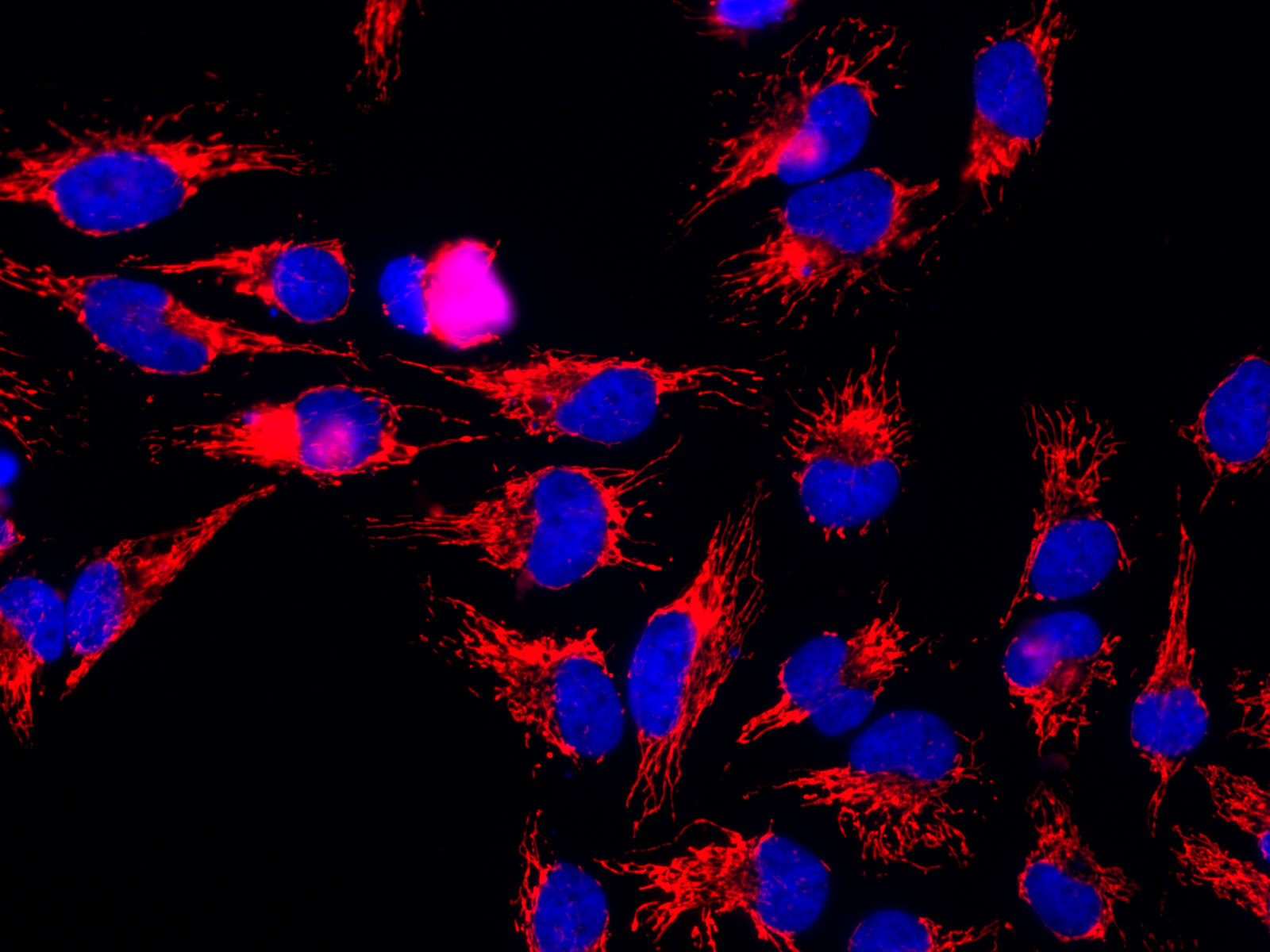
Molecular Biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and physical structure of biological macromolecules is known as molecular biology. Molecular biology was first described as an approach focused on the underpinnings of biological phenomena - uncovering the structures of biological molecules as well as their interactions, and how these interactions explain observations of classical biology. In 1945 the term molecular biology was used by physicist William Astbury. In 1953 Francis Crick, James Watson, Rosalind Franklin, and colleagues, working at Medical Research Council unit, Cavendish laboratory, Cambridge (now the MRC Laboratory of Molecular Biology), made a double helix model of DNA which changed the entire research scenario. They proposed the DNA structure based on previous research done by Ro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Chaperones
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermedia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GroEL
GroEL is a protein which belongs to the chaperonin family of molecular chaperones, and is found in many bacteria. It is required for the proper folding of many proteins. To function properly, GroEL requires the lid-like cochaperonin protein complex GroES. In eukaryotes the organellar proteins Hsp60 and Hsp10 are structurally and functionally nearly identical to GroEL and GroES, respectively, due to their endosymbiotic origin. HSP60 is implicated in mitochondrial protein import and macromolecular assembly. It may facilitate the correct folding of imported proteins, and may also prevent misfolding and promote the refolding and proper assembly of unfolded polypeptides generated under stress conditions in the mitochondrial matrix. HSP60 interacts with HRAS and with HBV protein X and HTLV-1 protein p40tax. HSP60 belongs to the chaperonin (HSP60) family. Note: This description may include information from UniProtKB. Alternate Names: 60 kDa chaperonin, Chaperonin 60, CPN60, Heat shock ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GroES
Heat shock 10 kDa protein 1 (Hsp10), also known as chaperonin 10 (cpn10) or early-pregnancy factor (EPF), is a protein that in humans is encoded by the ''HSPE1'' gene. The homolog in ''E. coli'' is GroES that is a chaperonin which usually works in conjunction with GroEL. Structure and function GroES exists as a ring-shaped oligomer of between six and eight identical subunits, while the 60 kDa chaperonin (cpn60 - or groEL in bacteria) forms a structure comprising 2 stacked rings, each ring containing 7 identical subunits. These ring structures assemble by self-stimulation in the presence of Mg2+-ATP. The central cavity of the cylindrical cpn60 tetradecamer provides an isolated environment for protein folding whilst cpn-10 binds to cpn-60 and synchronizes the release of the folded protein in an Mg2+-ATP dependent manner. The binding of cpn10 to cpn60 inhibits the weak ATPase activity of cpn60. ''Escherichia coli'' GroES has also been shown to bind ATP cooperatively, and wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DnaK
The 70 kilodalton heat shock proteins (Hsp70s or DnaK) are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an important part of the cell's machinery for protein folding, performing chaperoning functions, and helping to protect cells from the adverse effects of physiological stresses. Additionally, membrane-bound Hsp70s have been identified as a potential target for cancer therapies and their extracellularly localized counterparts have been identified as having both membrane-bound and membrane-free structures. Discovery Members of the Hsp70 family are very strongly upregulated by heat stress and toxic chemicals, particularly heavy metals such as arsenic, cadmium, copper, mercury, etc. Heat shock was originally discovered by Ferruccio Ritossa in the 1960s when a lab worker accidentally boosted the incubation temperature of Drosophila (fruit flies). When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DnaJ
In molecular biology, chaperone DnaJ, also known as Hsp40 (heat shock protein 40 kD), is a molecular chaperone protein. It is expressed in a wide variety of organisms from bacteria to humans. Function Molecular chaperones are a diverse family of proteins that function to protect proteins from irreversible aggregation during synthesis and in times of cellular stress. The bacterial molecular chaperone DnaK is an enzyme that couples cycles of ATP binding, hydrolysis, and ADP release by an N-terminal ATP-hydrolyzing domain to cycles of sequestration and release of unfolded proteins by a C-terminal substrate binding domain. Dimeric GrpE is the co-chaperone for DnaK, and acts as a nucleotide exchange factor, stimulating the rate of ADP release 5000-fold. DnaK is itself a weak ATPase; ATP hydrolysis by DnaK is stimulated by its interaction with another co-chaperone, DnaJ. Thus the co-chaperones DnaJ and GrpE are capable of tightly regulating the nucleotide-bound and substrate-bound st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GrpE
GrpE (''Gro-P'' like protein E) is a bacterial nucleotide exchange factor that is important for regulation of protein folding machinery, as well as the heat shock response. It is a heat-inducible protein and during stress it prevents unfolded proteins from accumulating in the cytoplasm. Accumulation of unfolded proteins in the cytoplasm can lead to cell death. Discovery GrpE is a nucleotide exchange factor that was first discovered by researchers in 1977 as a protein necessary to propagate bacteriophage λ, a virus that infects bacteria by highjacking the bacteria's own replication machinery, in ''Escherichia coli''. By using a genetic screen, researchers knocked out certain genes in E''. coli'' and then tested whether the bacteria was able to replicate, GrpE was found to be crucial to propagation. Since that time, GrpE has been identified in all bacteria and in Archaea where DnaK and DnaJ are present. The crystal structure of GrpE was determined in 1997 at 2.8 Angstrom and id ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperonin
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 belong to a large class of molecules that assist protein folding, called molecular chaperones. Newly made proteins usually must fold from a linear chain of amino acids into a three-dimensional tertiary structure. The energy to fold proteins is supplied by non-covalent interactions between the amino acid side chains of each protein, and by solvent effects. Most proteins spontaneously fold into their most stable three-dimensional conformation, which is usually also their functional conformation, but occasionally proteins mis-fold. Molecular chaperones catalyze protein refolding by accelerating partial unfolding of misfolded proteins, aided by energy supplied by the hydrolysis of adenosine triphosphate (ATP). Chaperonin proteins may also tag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Co-chaperone
Co-chaperones are proteins that assist chaperone (protein), chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly essential in stimulation of the ATPase activity of these chaperone proteins. There are a great number of different co-chaperones however based on their domain structure most of them fall into two groups: J-domain proteins and tetratricopeptide repeats (TPR). Co-chaperones assist heat shock proteins in the protein folding process. These co-chaperones can function in a number of ways. Primarily co-chaperones are involved in the ATPase functionality of their associated heat shock proteins. Co-chaperones catalyze the hydrolysis ATP to ADP on their respective chaperones which then allows them undergo a large conformational change that allows them to either bind to their substrates with higher affinity or aid in the release of the substrate followin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Chaperones
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermedia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |