|
Fluorinated
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a chemical compound, compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (F2, Cl2, Br2, I2). Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride. Organic chemistry Several pathways exist for the halogenation of organic compounds, including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate determines the pathway. The facility of halogenation is influenced by the halogen. Fluorine and chlorine are more elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers, e.g. polytetrafluoroethylene (PTFE). HF is widely used in the petrochemical industry as a component of superacids. Hydrogen fluoride boils at near room temperature, much higher than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid had been known in the glass industry before then. French chemist Edmond Frémy (1814–1894) is credited with discoveri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic Halogenation
In organic chemistry, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution. This organic reaction is typical of aromatic compounds and a very useful method for adding substituents to an aromatic system. : A few types of aromatic compounds, such as phenol, will react without a catalyst, but for typical benzene derivatives with less reactive substrates, a Lewis acid is required as a catalyst. Typical Lewis acid catalysts include , , and . These work by forming a highly electrophilic complex which is attacked by the benzene ring. Reaction mechanism The reaction mechanism for chlorination of benzene is the same as bromination of benzene. Iron(III) bromide and iron(III) chloride become inactivated if they react with water, including moisture in the air. Therefore, they are generated by adding iron filings to bromine or chlorine. Here is the mechanism of this reaction: : The mechanism for iodination is slightly different: iodine (I2) is treated wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Fluorination
Electrochemical fluorination (ECF), or electrofluorination, is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based organofluorine compounds.G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, B. McKusick "Fluorine Compounds, Organic" in "Ullmann’s Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. The general approach represents an application of electrosynthesis. The fluorinated chemical compounds produced by ECF are useful because of their distinctive solvation properties and the relative inertness of carbon–fluorine bonds. Two ECF synthesis routes are commercialized and commonly applied: the Simons process and the Phillips Petroleum process. It is also possible to electrofluorinate in various organic media.Fred G. Drakesmith "Electrofluorination of Organic Compounds" Topics in Current Chemistry,Vol. 193, Springer, Berlin-Heidelberg, 1997. Prior to the development of these methods, fluorination with fluorine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in 1825) and Antoine Jérôme Balard (in 1826), its name was derived from the Ancient Greek (bromos) meaning "stench", referring to its sharp and pungent smell. Elemental bromine is very reactive and thus does not occur as a native element in nature but it occurs in colourless soluble crystalline mineral halide salts, analogous to table salt. In fact, bromine and all the halogens are so reactive that they form bonds in pairs—never in single atoms. While it is rather rare in the Earth's crust, the high solubility of the bromide ion (Br) has caused its accumulation in the oceans. Commercial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Number
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek 'violet-coloured'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. It is the least abundant of the stable halogens, being the sixty-first most abundant element. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" (or "salt maker"). When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure. All of the halogens form acids when bonded to hydrogen. Most halogens are typically produced from minerals or salts. The middle halogens—chlorine, bromine, and iodine—are often used as disinfectants. Organobromides are the most important class of flame retardants, while elemental halogens are dangerous and can be toxic. History The fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
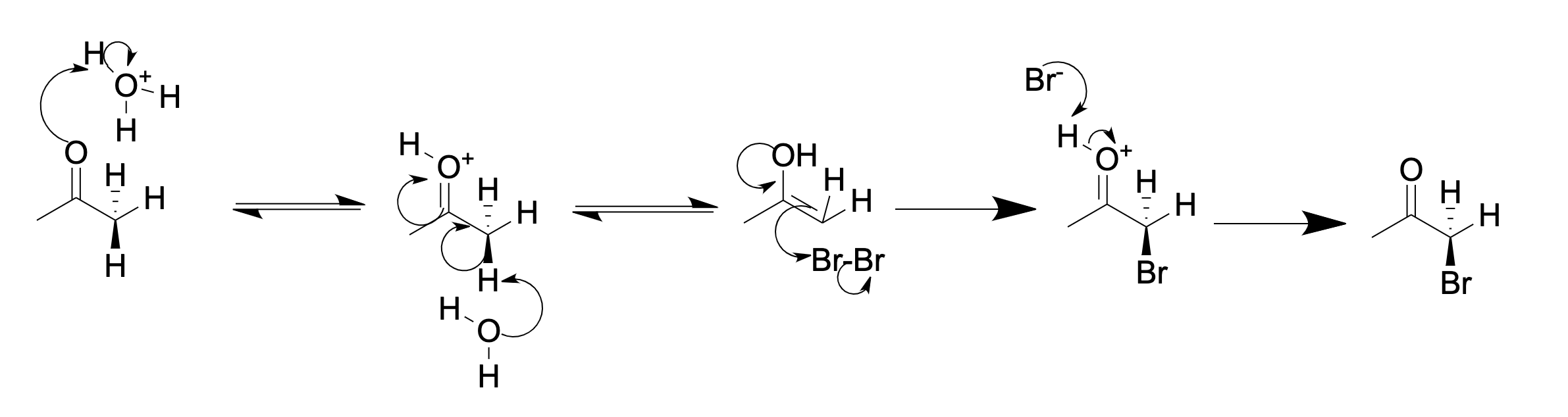
Ketone Halogenation
In organic chemistry, α-keto halogenation is a special type of halogenation. The reaction may be carried out under either acidic or basic conditions in an aqueous medium with the corresponding elemental halogen. In this way, chloride, bromide, and iodide (but notably not fluoride) functionality can be inserted selectively in the alpha position of a ketone. The position alpha to the carbonyl group in a ketone is easily halogenated. This is due to its ability to form an enolate in basic solution, or an enol in acidic solution. An example of alpha halogenation is the mono-bromination of acetone, carried out under either acidic or basic conditions, to give bromoacetone: Acidic (in acetic acid): Basic (in aqueous NaOH): In acidic solution, usually only one alpha hydrogen is replaced by a halogen, as each successive halogenation is slower than the first. The halogen decreases the basicity of the carbonyl oxygen, thus making protonation less favorable. However, in basic solut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkynes
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contributes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halothane Synthesis
Halothane, sold under the brand name Fluothane among others, is a general anaesthetic. It can be used to induce or maintain anaesthesia. One of its benefits is that it does not increase the production of saliva, which can be particularly useful in those who are difficult to intubate. It is given by inhalation. Side effects include an irregular heartbeat, respiratory depression, and hepatotoxicity. Like all volatile anesthetics, it should not be used in people with a personal or family history of malignant hyperthermia. It appears to be safe in porphyria. It is unclear whether use during pregnancy is harmful to the baby, and it is not generally recommended for use during a C-section. Halothane is a chiral molecule that is used as a racemic mixture. Halothane was discovered in 1955. It was approved for medical use in the United States in 1958. It is on the World Health Organization's List of Essential Medicines. Its use in developed countries has been mostly replaced by newer ane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloroethylene
The chemical compound trichloroethylene is a halocarbon commonly used as an industrial solvent. It is a clear, colourless non-flammable liquid with a chloroform-like sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as ''chlorothene''. The IUPAC name is trichloroethene. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. It has been sold under a variety of trade names. Under the trade names Trimar and Trilene, trichloroethylene was used as a volatile anesthetic and as an inhaled obstetrical analgesic in millions of patients. Groundwater and drinking water contamination from industrial discharge including trichloroethylene is a major concern for human health and has precipitated numerous incidents and lawsuits. History Pioneered by Imperial Chemical Industries in Britain, its development was hailed as an anesthetic revolution. Originally thought to possess less hepatotoxicity than chloroform, and without th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |