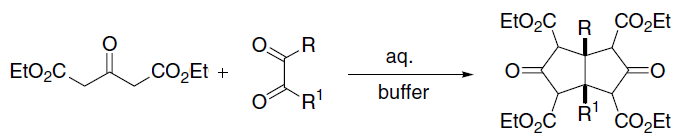
|
Ethyl Cyanoacetate
Ethyl cyanoacetate is an organic compound that contains a carboxylate ester and a nitrile. It is a colourless liquid with a pleasant odor. This material is useful as a starting material for synthesis due to its variety of functional groups and chemical reactivity. Production Ethyl cyanoacetate may be prepared in various ways: *Kolbe nitrile synthesis using ethyl chloroacetate and sodium cyanide. *Fischer esterification of cyanoacetic acid with ethanol in the presence of a strong mineral acids (e.g. concentrated sulfuric acid). The cyanoacetic acid can be prepared via Kolbe nitrile synthesis using sodium chloroacetate and sodium cyanide. *Reaction of the sodium cyanoacetate with ethyl bromide in an aqueous–organic two-phase system in the presence of a phase transfer catalyst. *Oxidation of 3-ethoxypropionitrile, an ether, with oxygen under pressure in the presence of cobalt(II) acetate tetrahydrate as catalyst and ''N''-hydroxyphthalimide as a radical generator. Properties Phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapour Pressure Cyano Acetic Acid Ethyl Ester
In physics, a vapor (American English) or vapour (British English and Canadian English; see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R. H. Petrucci, W. S. Harwood, and F. G. Herring, ''General Chemistry'', Prentice-Hall, 8th ed. 2002, p. 483–86. which means that the vapor can be condensed to a liquid by increasing the pressure on it without reducing the temperature. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas. For example, water has a critical temperature of , which is the highest temperature at which liquid water can exist. In the atmosphere at ordinary temperatures gaseous water (known as water vapor) will condense into a liquid if its partial pressure is increased sufficiently. A vapor may co-exist with a liquid (or a solid). When this is true, the two phases will be in equilibrium, and the gas-partial pressure will be equal to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Ethoxide
Sodium ethoxide, also referred to as sodium ethylate, is the ionic, organic compound with the formula , or NaOEt (Et = ethane). It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. It is commonly used as a strong base. Preparation Few procedures have been reported to prepare the anhydrous solid. Instead the material is typically prepared in a solution with ethanol. It is commercially available and as a solution in ethanol. It is easily prepared in the laboratory by treating sodium metal with absolute ethanol: : The reaction of sodium hydroxide with anhydrous ethanol suffers from incomplete conversion to the alkoxide. Structure The crystal structure of sodium ethoxide has been determined by X-ray crystallography. It consists of layers of alternating Na+ and O− centres with disordered ethyl groups covering the top and bottom of each layer. The ethyl layers pack back-to-back resulting in a lamellar structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strong Acids
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a hydron (chemistry), proton, H+, and an anion, A-. The Dissociation (chemistry), dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions. :HA -> H+ + A- Examples of strong acids are hydrochloric acid (HCl), perchloric acid (HClO4), nitric acid (HNO3) and sulfuric acid (H2SO4). A weak acid is only partially dissociated, with both the undissociated acid and its dissociation products being present, in solution, in Equilibrium chemistry, equilibrium with each other. :HA H+ + A- Acetic acid (CH3COOH) is an example of a weak acid. The strength of a weak acid is quantified by its acid dissociation constant, K_\ce value. The strength of a weak organic chemistry, organic acid may depend on substituent effects. The strength of an inorganic chemistry, inorganic acid is dependent on the oxidation state for the atom to which the prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Malonate
Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds such as barbiturates, artificial flavourings, vitamin B1, and vitamin B6. Structure and properties Malonic acid is a rather simple dicarboxylic acid, with two the carboxyl groups close together. In forming diethyl malonate from malonic acid, the hydroxyl group (−OH) on both of the carboxyl groups is replaced by an ethoxy group (−OEt; −OCH2CH3). The methylene group (−CH2−) in the middle of the malonic part of the diethyl malonate molecule is neighboured by two carbonyl groups (−C(=O)−). The hydrogen atoms on the carbon adjacent to the carbonyl group in a molecule is significantly more acidic than hydrogen atoms on a carbon adjacent to alkyl groups (up to 30 orders of magnitude). (This is known as the α position with re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonic Acid
Malonic acid (IUPAC systematic name: propanedioic acid) is a dicarboxylic acid with structure CH2(COOH)2. The ionized form of malonic acid, as well as its esters and salts, are known as malonates. For example, diethyl malonate is malonic acid's diethyl ester. The name originates from the Greek word μᾶλον (''malon'') meaning 'apple'. History Malonic acid is a naturally occurring substance found in many fruits and vegetables. There is a suggestion that citrus fruits produced in organic farming contain higher levels of malonic acid than fruits produced in conventional agriculture. Malonic acid was first prepared in 1858 by the French chemist Victor Dessaignes via the oxidation of malic acid. Structure and preparation The structure has been determined by X-ray crystallography and extensive property data including for condensed phase thermochemistry are available from the National Institute of Standards and Technology. A classical preparation of malonic acid starts from chlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Knoevenagel Condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence ''condensation''). The product is often an α,β-unsaturated ketone (a conjugated enone). In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form * or for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid. * , for instance nitromethane. where Z is an electron withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the aldehyde o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl sulfide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylene Bridge
In organic chemistry, a methylene bridge, methylene spacer, or methanediyl group is any part of a molecule with formula ; namely, a carbon atom bound to two hydrogen atoms and connected by single bonds to two other distinct atoms in the rest of the molecule. It is the repeating unit in the skeleton of the unbranched alkanes. A methylene bridge can also act as a bidentate ligand joining two metals in a coordination compound, such as titanium and aluminum in Tebbe's reagent.W. A. Herrmann (1982), "The methylene bridge". In ''Advances in Organometallic Chemistry'', volume 20, pages 195-197. A methylene bridge is often called a methylene group or simply methylene, as in "methylene chloride" (dichloromethane ). As a bridge in other compounds, for example in cyclic compounds, it is given the name methano. However, the term methylene group (or "methylidene") properly applies to the group when it is connected to the rest of the molecule by a double bond (), giving it chemical proper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |