|
Electron Spectrometer
An electron spectrometer is a device used to perform different forms of electron spectroscopy and electron microscopy. This requires analyzing the energy of an incoming beam of electrons. Most electron spectrometers use a hemispherical electron energy analyzer in which the beam of electrons is bent with electric or magnetic fields. Higher energy electrons will be bent less by the beam, this produces a spatially distributed range of energies. Electron spectrometers are used on a range of scientific equipment, including particle accelerators, transmission electron microscopes, and astronomical satellites. Types Electron spectrometers may determine electron energy based on time of flight, retarding potential (effectively a high-pass filter), resonant collision or curvature in a deflecting field (magnetic or electric). An electrostatic electron spectrometer uses the electric field, which cause electrons to move along field gradients, whereas magnetic devices cause electrons to move ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Spectroscopy
Electron spectroscopy refers to a group formed by techniques based on the analysis of the energies of emitted electrons such as Photoelectric effect, photoelectrons and Auger electrons. This group includes X-ray photoelectron spectroscopy (XPS), which also known as Electron Spectroscopy for Chemical Analysis (ESCA), Electron energy loss spectroscopy (EELS), Ultraviolet photoelectron spectroscopy (UPS), and Auger electron spectroscopy (AES). These analytical techniques are used to identify and determine the elements and their electronic structures from the surface of a test sample. Samples can be solids, gases or liquids.Yang Leng; ''Materials Characterization: Introduction to Microscopic and Spectroscopic Methods (Second Edition)''; Publisher John Wiley & Sons, Incorporated 2013; p: 191-192, 221-224.Daintith, J.; ''Dictionary of Chemistry (6th Edition)''; Oxford University Press, 2008; p: 191, 416, 541 Chemical information is obtained only from the uppermost atomic layers of the sam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemispherical Electron Energy Analyzer
A hemispherical electron energy analyzer or hemispherical deflection analyzer is a type of electron energy spectrometer generally used for applications where high energy resolution is needed—different varieties of electron spectroscopy such as angle-resolved photoemission spectroscopy (ARPES), X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) or in imaging applications such as photoemission electron microscopy (PEEM) and low-energy electron microscopy (LEEM). It consists of two concentric conductive hemispheres that serve as electrodes that bend the trajectories of the electrons entering a narrow slit at one end so that their final radii depend on their kinetic energy. The analyzer, therefore, provides a mapping from kinetic energies to positions on a detector. Function An ideal hemispherical analyzer consists of two concentric hemispherical electrodes (inner and outer hemispheres) of radii R_ and R_ held at proper voltages. In such a system, the ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Beam
Cathode rays or electron beam (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode (the electrode connected to the negative terminal of the voltage supply). They were first observed in 1859 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein ''Kathodenstrahlen'', or cathode rays. In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the ''electron''. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen. Description Cathode rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. To release electrons into the tube, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Time-of-flight Mass Spectrometry
Time-of-flight mass spectrometry (TOFMS) is a method of mass spectrometry in which an ion's mass-to-charge ratio is determined by a time of flight measurement. Ions are accelerated by an electric field of known strength. This acceleration results in an ion having the same kinetic energy as any other ion that has the same charge. The velocity of the ion depends on the mass-to-charge ratio (heavier ions of the same charge reach lower speeds, although ions with higher charge will also increase in velocity). The time that it subsequently takes for the ion to reach a detector at a known distance is measured. This time will depend on the velocity of the ion, and therefore is a measure of its mass-to-charge ratio. From this ratio and known experimental parameters, one can identify the ion. Theory The potential energy of a charged particle in an electric field is related to the charge of the particle and to the strength of the electric field: where ''E''p is potential energy, ''q'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionized, for example by bombarding it with a beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragments) are then separated accordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Filtered Transmission Electron Microscopy
Energy-filtered transmission electron microscopy (EFTEM) is a technique used in transmission electron microscopy, in which only electrons of particular kinetic energies are used to form the image or diffraction pattern. The technique can be used to aid chemical analysis of the sample in conjunction with complementary techniques such as electron crystallography. Principle If a very thin sample is illuminated with a beam of high-energy electrons, then a majority of the electrons will pass unhindered through the sample but some will interact with the sample, being scattered elastically or inelastically (phonon scattering, plasmon scattering or inner shell ionisation). Inelastic scattering results in both a loss of energy and a change in momentum, which in the case of inner shell ionisation is characteristic of the element in the sample. If the electron beam emerging from the sample is passed through a magnetic prism, then the flight path of the electrons will vary depending on their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PEEM
Photoemission electron microscopy (PEEM, also called photoelectron microscopy, PEM) is a type of electron microscopy that utilizes local variations in electron emission to generate image contrast. The excitation is usually produced by ultraviolet light, synchrotron radiation or X-ray sources. PEEM measures the coefficient indirectly by collecting the emitted secondary electrons generated in the electron cascade that follows the creation of the primary core hole in the absorption process. PEEM is a surface sensitive technique because the emitted electrons originate from a shallow layer. In physics, this technique is referred to as PEEM, which goes together naturally with low-energy electron diffraction (LEED), and low-energy electron microscopy ( LEEM). In biology, it is called photoelectron microscopy (PEM), which fits with photoelectron spectroscopy (PES), transmission electron microscopy (TEM), and scanning electron microscopy (SEM). History Initial development In 1933, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Energy Loss Spectroscopy
In electron energy loss spectroscopy (EELS) a material is exposed to a beam of electrons with a known, narrow range of kinetic energies. Some of the electrons will undergo inelastic scattering, which means that they lose energy and have their paths slightly and randomly deflected. The amount of energy loss can be measured via an electron spectrometer and interpreted in terms of what caused the energy loss. Inelastic interactions include phonon excitations, inter- and intra-band transitions, plasmon excitations, inner shell ionizations, and Cherenkov radiation. The inner-shell ionizations are particularly useful for detecting the elemental components of a material. For example, one might find that a larger-than-expected number of electrons comes through the material with 285 eV less energy than they had when they entered the material. This is approximately the amount of energy needed to remove an inner-shell electron from a carbon atom, which can be taken as evidence ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
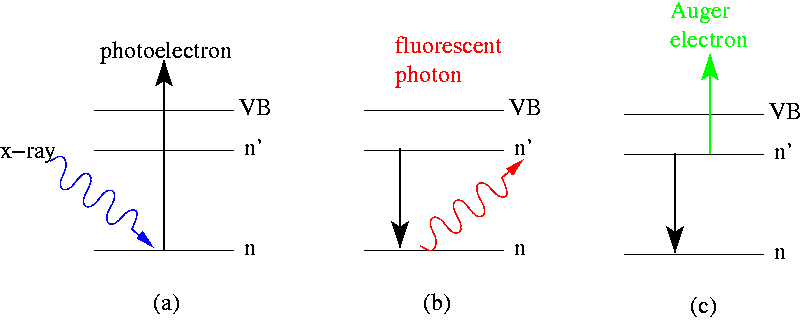
Auger Electron Spectroscopy
file:HD.6C.037 (11856519893).jpg, A Hanford Site, Hanford scientist uses an Auger electron spectrometer to determine the elemental composition of surfaces. Auger electron spectroscopy (AES; pronounced in French) is a common analytical technique used specifically in the study of surface science, surfaces and, more generally, in the area of materials science. It is a form of electron spectroscopy that relies on the Auger effect, based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Victor Auger, Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal ''European Physical Journal, Zeitschrift für Physik'' in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angle-resolved Photoemission Spectroscopy
Angle-resolved photoemission spectroscopy (ARPES) is an experimental technique used in condensed matter physics to probe the allowed energies and momenta of the electrons in a material, usually a crystalline solid. It is based on the photoelectric effect, in which an incoming photon of sufficient energy ejects an electron from the surface of a material. By directly measuring the kinetic energy and emission angle distributions of the emitted photoelectrons, the technique can map the electronic band structure and Fermi surfaces. ARPES is best suited for the study of one- or two-dimensional materials. It has been used by physicists to investigate high-temperature superconductors, graphene, Topological insulator, topological materials, quantum well states, and materials exhibiting charge density waves. ARPES systems consist of a monochromatic light source to deliver a narrow beam of photons, a sample holder connected to a manipulator used to position the sample of a material, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin Polarization
Spin polarization is the degree to which the spin, i.e., the intrinsic angular momentum of elementary particles, is aligned with a given direction. This property may pertain to the spin, hence to the magnetic moment, of conduction electrons in ferromagnetic metals, such as iron, giving rise to spin-polarized currents. It may refer to (static) spin waves, preferential correlation of spin orientation with ordered lattices (semiconductors or insulators). It may also pertain to beams of particles, produced for particular aims, such as polarized neutron scattering or muon spin spectroscopy. Spin polarization of electrons or of nuclei, often called simply magnetization, is also produced by the application of a magnetic field. Curie law is used to produce an induction signal in Electron spin resonance (ESR or EPR) and in Nuclear magnetic resonance (NMR). Spin polarization is also important for spintronics, a branch of electronics. Magnetic semiconductors are being researched as possib ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Optics
Electron optics is a mathematical framework for the calculation of electron trajectories along electromagnetic fields. The term ''optics'' is used because magnetic and electrostatic lenses act upon a charged particle beam similarly to optical lenses upon a light beam. Electron optics calculations are crucial for the design of electron microscopes and particle accelerators. In the paraxial approximation, trajectory calculations can be carried out using ray transfer matrix analysis. Electron properties Electrons are charged particles (point charges with rest mass) with spin 1/2 (hence they are fermions). Electrons can be accelerated by suitable electric (or magnetic) fields, thereby acquiring kinetic energy. Given sufficient voltage, the electron can be accelerated sufficiently fast to exhibit measurable relativistic effects. According to wave particle duality, electrons can also be considered as matter waves with properties such as wavelength, phase and amplitude. Geometric e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)

