|
Epithelial Cell Adhesion Molecule
Epithelial cell adhesion molecule (EpCAM), also known as CD326 among other names, is a transmembrane glycoprotein mediating Ca2+-independent homotypic cell–cell adhesion in epithelia. EpCAM is also involved in cell signaling, migration, proliferation, and differentiation. Additionally, EpCAM has oncogenic potential via its capacity to upregulate c-myc, e-fabp, and cyclins A & E. Since EpCAM is expressed exclusively in epithelia and epithelial-derived neoplasms, EpCAM can be used as diagnostic marker for various cancers. It appears to play a role in tumorigenesis and metastasis of carcinomas, so it can also act as a potential prognostic marker and as a potential target for immunotherapeutic strategies. Expression pattern First discovered in 1979, EpCAM was initially described as a dominant surface antigen on human colon carcinoma. Because of its prevalence on many carcinomas, it has been "discovered" many different times. EpCAM therefore has many aliases the most notable of w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
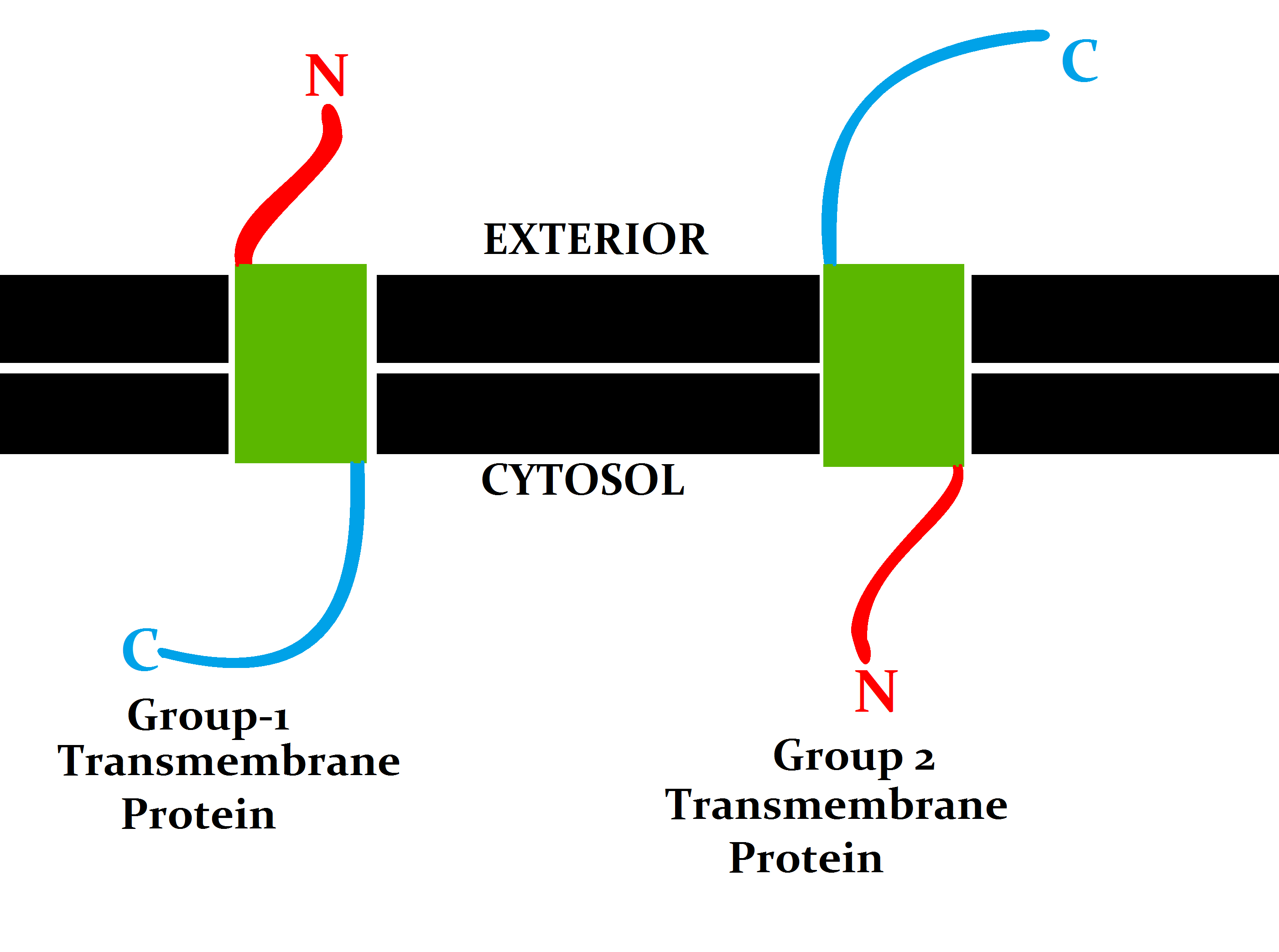
Transmembrane
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them ( beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gastrointestinal Tract
The gastrointestinal tract (GI tract, digestive tract, alimentary canal) is the tract or passageway of the digestive system that leads from the mouth to the anus. The GI tract contains all the major organs of the digestive system, in humans and other animals, including the esophagus, stomach, and intestines. Food taken in through the mouth is digested to extract nutrients and absorb energy, and the waste expelled at the anus as feces. ''Gastrointestinal'' is an adjective meaning of or pertaining to the stomach and intestines. Most animals have a "through-gut" or complete digestive tract. Exceptions are more primitive ones: sponges have small pores (ostia) throughout their body for digestion and a larger dorsal pore ( osculum) for excretion, comb jellies have both a ventral mouth and dorsal anal pores, while cnidarians and acoels have a single pore for both digestion and excretion. The human gastrointestinal tract consists of the esophagus, stomach, and intestines, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epidermal Growth Factor
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-k Da and has 53 amino acid residues and three intramolecular disulfide bonds. EGF was originally described as a secreted peptide found in the submaxillary glands of mice and in human urine. EGF has since been found in many human tissues, including platelets, submandibular gland (submaxillary gland), and parotid gland. Initially, human EGF was known as urogastrone. Structure In humans, EGF has 53 amino acids (sequence NSDSECPLSHDGYCLHDGVCMYIEALDKYACNCVVGYIGERCQYRDLKWWELR), with a molecular mass of around 6 kDa. It contains three disulfide bridges (Cys6-Cys20, Cys14-Cys31, Cys33-Cys42). Function EGF, via binding to its cognate receptor, results in cellular proliferation, differentiation, and survival. Salivary EGF, which seems to be regulated by dietary inorganic iodine, also plays an important physiological role in the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component ( water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylated
Glycosylation is the reaction in which a carbohydrate (or 'glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction (though in practice, 'glycation' often refers more specifically to Maillard-type reactions). Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein used by the immune system to identify and neutralize foreign objects such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen. Each tip of the "Y" of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can ''tag'' a microbe or an infected cell for attack by other parts of the immune system, or can neutralize it directly (for example, by blocking a part of a virus that is essential for its invasion). To allow the immune system to recognize millions of different antigens, the antigen-binding sites at both tips of the antibody come in an equally wide variety. In contrast, the remainder of the antibody is relatively constant. It only occurs in a few varian ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selectins
The selectins (cluster of differentiation 62 or CD62) are a family of cell adhesion molecules (or CAMs). All selectins are single-chain transmembrane glycoproteins that share similar properties to C-type lectins due to a related amino terminus and calcium-dependent binding. Selectins bind to sugar moieties and so are considered to be a type of lectin, cell adhesion proteins that bind sugar polymers. Structure All three known members of the selectin family (L-, E-, and P-selectin) share a similar cassette structure: an N-terminal, calcium-dependent lectin domain, an epidermal growth factor (EGF)-like domain, a variable number of consensus repeat units (2, 6, and 9 for L-, E-, and P-selectin, respectively), a transmembrane domain (TM) and an intracellular cytoplasmic tail (cyto). The transmembrane and cytoplasmic parts are not conserved across the selectins being responsible for their targeting to different compartments. Though they share common elements, their tissue distribut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrins
Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface (''e.g''. signal platelets to initiate an interaction with coagulation factors). Several types of integrins exist, and one cell generally has multiple different types on its surface. Integrins are found in all animals while integrin-like receptors are found in plant cells. Integrins work alongside other proteins such as cadherins, the immunoglobulin superfamily cell adhesion molecules, selectins and syndecans, to mediate cell–cell and cell–matrix interaction. Ligands for integrins include fibronectin, vitronectin, collagen and laminin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadherins
Cadherins (named for "calcium-dependent adhesion") are a type of cell adhesion molecule (CAM) that is important in the formation of adherens junctions to allow cells to adhere to each other . Cadherins are a class of type-1 transmembrane proteins, and they are dependent on calcium (Ca2+) ions to function, hence their name. Cell-cell adhesion is mediated by extracellular cadherin domains, whereas the intracellular cytoplasmic tail associates with numerous adaptors and signaling proteins, collectively referred to as the cadherin adhesome. The cadherin family is essential in maintaining the cell-cell contact and regulating cytoskeletal complexes. The cadherin superfamily includes cadherins, protocadherins, desmogleins, desmocollins, and more. In structure, they share ''cadherin repeats'', which are the extracellular Ca2+-binding domains. There are multiple classes of cadherin molecules, each designated with a prefix (in general, noting the types of tissue with which it is associated). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Adhesion Molecules
Cell adhesion molecules (CAMs) are a subset of cell surface proteins that are involved in the binding of cells with other cells or with the extracellular matrix (ECM), in a process called cell adhesion. In essence, CAMs help cells stick to each other and to their surroundings. CAMs are crucial components in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in generating force and movement and consequently ensuring that organs are able to execute their functions normally. In addition to serving as "molecular glue", CAMs play important roles in the cellular mechanisms of growth, contact inhibition, and apoptosis. Aberrant expression of CAMs may result in a wide range of pathologies, ranging from frostbite to cancer. Structure CAMs are typically single-pass transmembrane receptors and are composed of three conserved domains: an intracellular domain that interacts with the cytoskeleton, a transmembrane domain, and an extrac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prostate Cancer
Prostate cancer is cancer of the prostate. Prostate cancer is the second most common cancerous tumor worldwide and is the fifth leading cause of cancer-related mortality among men. The prostate is a gland in the male reproductive system that surrounds the urethra just below the bladder. It is located in the hypogastric region of the abdomen. To give an idea of where it is located, the bladder is superior to the prostate gland as shown in the image The rectum is posterior in perspective to the prostate gland and the ischial tuberosity of the pelvic bone is inferior. Only those who have male reproductive organs are able to get prostate cancer. Most prostate cancers are slow growing. Cancerous cells may spread to other areas of the body, particularly the bones and lymph nodes. It may initially cause no symptoms. In later stages, symptoms include pain or difficulty urinating, blood in the urine, or pain in the pelvis or back. Benign prostatic hyperplasia may produce similar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Androgen
An androgen (from Greek ''andr-'', the stem of the word meaning "man") is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This includes the embryological development of the primary male sex organs, and the development of male secondary sex characteristics at puberty. Androgens are synthesized in the testes, the ovaries, and the adrenal glands. Androgens increase in both males and females during puberty. The major androgen in males is testosterone. Dihydrotestosterone (DHT) and androstenedione are of equal importance in male development. DHT ''in utero'' causes differentiation of the penis, scrotum and prostate. In adulthood, DHT contributes to balding, prostate growth, and sebaceous gland activity. Although androgens are commonly thought of only as male sex hormones, females also have them, but at lower levels: they function in libido and sexual arousal. Al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |